The poly(A) binding protein is internalized in virus-induced vesicles or redistributed to the nucleolus during turnip mosaic virus infection
- PMID: 17670821
- PMCID: PMC2045535
- DOI: 10.1128/JVI.01243-07
The poly(A) binding protein is internalized in virus-induced vesicles or redistributed to the nucleolus during turnip mosaic virus infection
Abstract
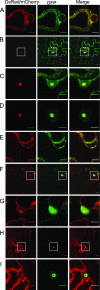
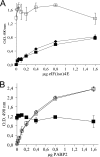
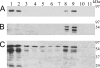
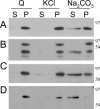
Poly(A) binding protein 2 (PABP2) of Arabidopsis thaliana was previously shown to interact with VPg-Pro of turnip mosaic virus (TuMV) and may consequently play an important role during infection. Subcellular fractionation experiments revealed that PABP2 was predominantly a cytoplasmic soluble protein in healthy plants. However, in TuMV-infected plants, a subpopulation of PABP2 was membrane associated or was localized in the nucleus. Confocal microscopy experiments indicated that PABP2 was partially retargeted to the nucleolus in the presence of TuMV VPg-Pro. In addition, the membrane association of PABP2 during TuMV infection resulted from the internalization of the host protein in 6K-VPg-Pro-induced vesicles, as shown by a combination of confocal microscopy and sucrose gradient fractionation experiments. This redistribution of an important translation initiation factor to the nucleolus and to membrane structure likely underlies two important processes of the TuMV replication cycle.
Figures




Similar articles
-
Heat shock 70 protein interaction with Turnip mosaic virus RNA-dependent RNA polymerase within virus-induced membrane vesicles.Virology. 2008 Apr 25;374(1):217-27. doi: 10.1016/j.virol.2007.12.014. Epub 2008 Jan 28. Virology. 2008. PMID: 18222516
-
Eukaryotic elongation factor 1A interacts with Turnip mosaic virus RNA-dependent RNA polymerase and VPg-Pro in virus-induced vesicles.Virology. 2008 Jul 20;377(1):216-25. doi: 10.1016/j.virol.2008.04.015. Epub 2008 May 23. Virology. 2008. PMID: 18501944
-
Arabidopsis thaliana class II poly(A)-binding proteins are required for efficient multiplication of turnip mosaic virus.J Gen Virol. 2008 Sep;89(Pt 9):2339-2348. doi: 10.1099/vir.0.2008/002139-0. J Gen Virol. 2008. PMID: 18753244
-
Involvement of the plant nucleolus in virus and viroid infections: parallels with animal pathosystems.Adv Virus Res. 2010;77:119-58. doi: 10.1016/B978-0-12-385034-8.00005-3. Adv Virus Res. 2010. PMID: 20951872 Free PMC article. Review.
-
How does the stressed out ER find relief during virus infection?Curr Opin Virol. 2016 Apr;17:74-79. doi: 10.1016/j.coviro.2016.01.018. Epub 2016 Feb 9. Curr Opin Virol. 2016. PMID: 26871502 Review.
Cited by
-
Ribosomal protein P0 promotes Potato virus A infection and functions in viral translation together with VPg and eIF(iso)4E.J Virol. 2013 Apr;87(8):4302-12. doi: 10.1128/JVI.03198-12. Epub 2013 Jan 30. J Virol. 2013. PMID: 23365448 Free PMC article.
-
Turnip mosaic virus NIb weakens the function of eukaryotic translation initiation factor 6 facilitating viral infection in Nicotiana benthamiana.Mol Plant Pathol. 2024 Feb;25(2):e13434. doi: 10.1111/mpp.13434. Mol Plant Pathol. 2024. PMID: 38388027 Free PMC article.
-
Imaging Techniques to Study Plant Virus Replication and Vertical Transmission.Viruses. 2021 Feb 25;13(3):358. doi: 10.3390/v13030358. Viruses. 2021. PMID: 33668729 Free PMC article. Review.
-
Turnip Mosaic Virus Uses the SNARE Protein VTI11 in an Unconventional Route for Replication Vesicle Trafficking.Plant Cell. 2018 Oct;30(10):2594-2615. doi: 10.1105/tpc.18.00281. Epub 2018 Aug 27. Plant Cell. 2018. PMID: 30150314 Free PMC article.
-
Cylindrical Inclusion Protein of Turnip Mosaic Virus Serves as a Docking Point for the Intercellular Movement of Viral Replication Vesicles.Plant Physiol. 2017 Dec;175(4):1732-1744. doi: 10.1104/pp.17.01484. Epub 2017 Oct 31. Plant Physiol. 2017. PMID: 29089395 Free PMC article.
References
-
- Barneche, F., F. Steinmetz, and M. Echeverria. 2000. Fibrillarin genes encode both a conserved nucleolar protein and a novel small nucleolar RNA involved in ribosomal RNA methylation in Arabidopsis thaliana. J. Biol. Chem. 275:27212-27220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

