Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein
- PMID: 17670828
- PMCID: PMC2045520
- DOI: 10.1128/JVI.01343-07
Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein
Abstract
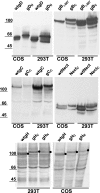
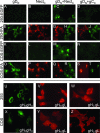
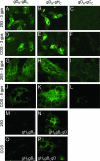
The interactions between herpes simplex virus gD and its nectin1 receptor or between gD, gB, and gH were analyzed by complementation of the N and C portions of split enhanced green fluorescent protein (EGFP) fused to the glycoproteins. The gD(N)-Nect(C) complex was readily detected; the gD(N)-gC(C) complex was undetectable, highlighting the specificity of the assay. Split EGFP complementation was detected between proteins designated gD(N)+gH(C), gD(N)+gB(C), and gH(N)+gB(C)+wtgD (gB was deleted of endocytosis motifs), both in cells transfected with two-tree glycoproteins and in syncytia. The in situ assay provides evidence that gD interacts with gH and gB independently of each other and supports a model whereby gH and gB in complex exert their activities to gD.
Figures
References
-
- Avitabile, E., G. Lombardi, T. Gianni, M. Capri, and G. Campadelli-Fiume. 2004. Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wild-type gB or an endocytosis-defective gB mutant and downmodulates their cell surface expression. J. Virol. 78:8015-8025. - PMC - PubMed
-
- Campadelli-Fiume, G., M. Amasio, E. Avitabile, A. Cerretani, C. Forghieri, T. Gianni, and L. Menotti. 2007. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev. Med. Virol. 17:313-326. - PubMed
-
- Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin superfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

