Serine/arginine-rich proteins contribute to negative regulator of splicing element-stimulated polyadenylation in rous sarcoma virus
- PMID: 17670832
- PMCID: PMC2045511
- DOI: 10.1128/JVI.00919-07
Serine/arginine-rich proteins contribute to negative regulator of splicing element-stimulated polyadenylation in rous sarcoma virus
Abstract
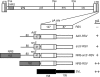
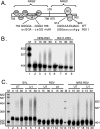
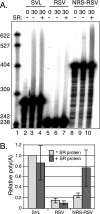
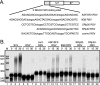
Rous sarcoma virus (RSV) requires large amounts of unspliced RNA for replication. Splicing and polyadenylation are coupled in the cells they infect, which raises the question of how viral RNA is efficiently polyadenylated in the absence of splicing. Optimal RSV polyadenylation requires a far-upstream splicing control element, the negative regulator of splicing (NRS), that binds SR proteins and U1/U11 snRNPs and functions as a pseudo-5' splice site that interacts with and sequesters 3' splice sites. We investigated a link between NRS-mediated splicing inhibition and efficient polyadenylation. In vitro, the NRS alone activated a model RSV polyadenylation substrate, and while the effect did not require the snRNP-binding sites or a downstream 3' splice site, SR proteins were sufficient to stimulate polyadenylation. Consistent with this, SELEX-binding sites for the SR proteins ASF/SF2, 9G8, and SRp20 were able to stimulate polyadenylation when placed upstream of the RSV poly(A) site. In vivo, however, the SELEX sites improved polyadenylation in proviral clones only when the NRS-3' splice site complex could form. Deletions that positioned the SR protein-binding sites closer to the poly(A) site eliminated the requirement for the NRS-3' splice site interaction. This indicates a novel role for SR proteins in promoting RSV polyadenylation in the context of the NRS-3' splice site complex, which is thought to bridge the long distance between the NRS and poly(A) site. The results further suggest a more general role for SR proteins in polyadenylation of cellular mRNAs.
Figures





Similar articles
-
RNA processing control in avian retroviruses.Front Biosci. 2008 May 1;13:3869-83. doi: 10.2741/2975. Front Biosci. 2008. PMID: 18508481 Free PMC article. Review.
-
Efficient polyadenylation of Rous sarcoma virus RNA requires the negative regulator of splicing element.Nucleic Acids Res. 2002 Feb 1;30(3):810-7. doi: 10.1093/nar/30.3.810. Nucleic Acids Res. 2002. PMID: 11809895 Free PMC article.
-
Juxtaposition of two distant, serine-arginine-rich protein-binding elements is required for optimal polyadenylation in Rous sarcoma virus.J Virol. 2011 Nov;85(21):11351-60. doi: 10.1128/JVI.00721-11. Epub 2011 Aug 17. J Virol. 2011. PMID: 21849435 Free PMC article.
-
Evidence that a threshold of serine/arginine-rich (SR) proteins recruits CFIm to promote rous sarcoma virus mRNA 3' end formation.Virology. 2016 Nov;498:181-191. doi: 10.1016/j.virol.2016.08.021. Epub 2016 Sep 4. Virology. 2016. PMID: 27596537 Free PMC article.
-
The structure and function of the rous sarcoma virus RNA stability element.J Cell Biochem. 2011 Nov;112(11):3085-92. doi: 10.1002/jcb.23272. J Cell Biochem. 2011. PMID: 21769913 Free PMC article. Review.
Cited by
-
Long-distance regulation of Add2 pre-mRNA3'end processing.RNA Biol. 2013 Apr;10(4):516-27. doi: 10.4161/rna.23855. Epub 2013 Feb 14. RNA Biol. 2013. PMID: 23411391 Free PMC article.
-
SRSF3 and SRSF7 modulate 3'UTR length through suppression or activation of proximal polyadenylation sites and regulation of CFIm levels.Genome Biol. 2021 Mar 11;22(1):82. doi: 10.1186/s13059-021-02298-y. Genome Biol. 2021. PMID: 33706811 Free PMC article.
-
RNA processing control in avian retroviruses.Front Biosci. 2008 May 1;13:3869-83. doi: 10.2741/2975. Front Biosci. 2008. PMID: 18508481 Free PMC article. Review.
-
Polypyrimidine tract binding protein prevents activity of an intronic regulatory element that promotes usage of a composite 3'-terminal exon.J Biol Chem. 2009 Nov 20;284(47):32370-83. doi: 10.1074/jbc.M109.029314. Epub 2009 Sep 17. J Biol Chem. 2009. PMID: 19762469 Free PMC article.
-
Molecular mechanisms of eukaryotic pre-mRNA 3' end processing regulation.Nucleic Acids Res. 2010 May;38(9):2757-74. doi: 10.1093/nar/gkp1176. Epub 2009 Dec 30. Nucleic Acids Res. 2010. PMID: 20044349 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

