The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations
- PMID: 17670839
- PMCID: PMC2045536
- DOI: 10.1128/JVI.01033-07
The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations
Abstract
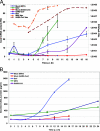
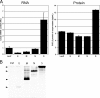
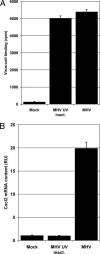
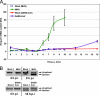
Murine hepatitis virus (MHV) and severe acute respiratory syndrome (SARS) coronavirus (CoV) are two of the best-studied representatives of the family Coronaviridae. During CoV infection, numerous cytokines and chemokines are induced in vitro and in vivo. Human interleukin 8 and its mouse functional counterpart, CXCL2, are early-expressed chemokines. Here we show that SARS-CoV and MHV induce endoplasmic reticulum (ER) stress and Cxcl2 mRNA transcription during infection in vitro. Expression of the viral spike protein significantly induced ER stress and Cxcl2 mRNA upregulation, while expression of the other structural genes did not. Additional experiments with UV-inactivated virus, cell-cell fusion-blocking antibodies, and an MHV mutant with a defect in spike protein maturation demonstrated that spike-host interactions in the ER are responsible for the induction of ER stress and subsequent Cxcl2 mRNA transcription. Despite significant increases in levels of Cxcl2 mRNA and functional nucleus-to-cytoplasm RNA transport, no CXCL2 protein was released into the medium from MHV-infected cells. Yet Sendai virus-infected cells showed substantial Cxcl2 mRNA induction and a simultaneous increase in levels of secreted CXCL2 protein. Our results demonstrate that expression of CoV spike proteins induces ER stress, which could subsequently trigger innate immune responses. However, at that point in infection, translation of host mRNA is already severely reduced in infected cells, preventing the synthesis of CXCL2 and ER stress proteins despite their increased mRNA concentrations.
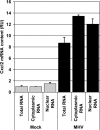
Figures



References
-
- Bensaude, E., J. L. Turner, P. R. Wakeley, D. A. Sweetman, C. Pardieu, T. W. Drew, T. Wileman, and P. P. Powell. 2004. Classical swine fever virus induces proinflammatory cytokines and tissue factor expression and inhibits apoptosis and interferon synthesis during the establishment of long-term infection of porcine vascular endothelial cells. J. Gen. Virol. 85:1029-1037. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

