Macrorheology and adaptive microrheology of endothelial cells subjected to fluid shear stress
- PMID: 17670893
- PMCID: PMC3251213
- DOI: 10.1152/ajpcell.00193.2007
Macrorheology and adaptive microrheology of endothelial cells subjected to fluid shear stress
Abstract
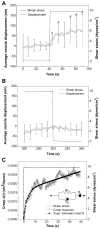
Vascular endothelial cells (ECs) respond to temporal and spatial characteristics of hemodynamic forces by alterations in their adhesiveness to leukocytes, secretion of vasodilators, and permeability to blood-borne constituents. These physiological and pathophysiological changes are tied to adaptation of cell mechanics and mechanotransduction, the process by which cells convert forces to intracellular biochemical signals. The exact time scales of these mechanical adaptations, however, remain unknown. We used particle-tracking microrheology to study adaptive changes in intracellular mechanics in response to a step change in fluid shear stress, which simulates both rapid temporal and steady features of hemodynamic forces. Results indicate that ECs become significantly more compliant as early as 30 s after a step change in shear stress from 0 to 10 dyn/cm(2) followed by recovery of viscoelastic parameters within 4 min of shearing, even though shear stress was maintained. After ECs were sheared for 5 min, return of shear stress to 0 dyn/cm(2) in a stepwise manner did not result in any further rheological adaptation. Average vesicle displacements were used to determine time-dependent cell deformation and macrorheological parameters by fitting creep function to a linear viscoelastic liquid model. Characteristic time and magnitude for shear-induced deformation were 3 s and 50 nm, respectively. We conclude that ECs rapidly adapt their mechanical properties in response to shear stress, and we provide the first macrorheological parameters for time-dependent deformations of ECs to a physiological forcing function. Such studies provide insight into pathologies such as atherosclerosis, which may find their origins in EC mechanics.
Figures


Similar articles
-
A model for shear stress-induced deformation of a flow sensor on the surface of vascular endothelial cells.J Theor Biol. 2001 May 21;210(2):221-36. doi: 10.1006/jtbi.2001.2290. J Theor Biol. 2001. PMID: 11371176
-
A model for shear stress sensing and transmission in vascular endothelial cells.Biophys J. 2003 Jun;84(6):4087-101. doi: 10.1016/S0006-3495(03)75134-0. Biophys J. 2003. PMID: 12770912 Free PMC article.
-
Vascular mechanobiology: endothelial cell responses to fluid shear stress.Circ J. 2009 Nov;73(11):1983-92. doi: 10.1253/circj.cj-09-0583. Epub 2009 Oct 5. Circ J. 2009. PMID: 19801852 Review.
-
Shear-stress preconditioning and tissue-engineering-based paradigms for generating arterial substitutes.Biotechnol Appl Biochem. 2004 Apr;39(Pt 2):151-7. doi: 10.1042/BA20030148. Biotechnol Appl Biochem. 2004. PMID: 15032735
-
Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype.J Appl Physiol (1985). 2008 Mar;104(3):588-600. doi: 10.1152/japplphysiol.01096.2007. Epub 2007 Dec 6. J Appl Physiol (1985). 2008. PMID: 18063803 Free PMC article. Review.
Cited by
-
Mechanobiology of dynamic enzyme systems.APL Bioeng. 2020 Mar 3;4(1):010907. doi: 10.1063/1.5133645. eCollection 2020 Mar. APL Bioeng. 2020. PMID: 32161834 Free PMC article.
-
β1-Integrin-Mediated Adhesion Is Lipid-Bilayer Dependent.Biophys J. 2017 Sep 5;113(5):1080-1092. doi: 10.1016/j.bpj.2017.07.010. Biophys J. 2017. PMID: 28877491 Free PMC article.
-
Mechanotransduction in Endothelial Cells in Vicinity of Cancer Cells.Cell Mol Bioeng. 2022 Jul 5;15(4):313-330. doi: 10.1007/s12195-022-00728-w. eCollection 2022 Aug. Cell Mol Bioeng. 2022. PMID: 36119131 Free PMC article.
-
A microfluidic chamber-based approach to map the shear moduli of vascular cells and other soft materials.Sci Rep. 2017 May 23;7(1):2305. doi: 10.1038/s41598-017-02659-3. Sci Rep. 2017. PMID: 28536424 Free PMC article.
-
Low intensity ultrasound perturbs cytoskeleton dynamics.Soft Matter. 2012 Feb 28;8(8):2438-2443. doi: 10.1039/C2SM07246G. Soft Matter. 2012. PMID: 23646063 Free PMC article.
References
-
- Butler PJ, Norwich G, Weinbaum S, Chien S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am J Physiol Cell Physiol. 2001;280:C962–C969. - PubMed
-
- Butler PJ, Tsou TC, Li JYS, Usami S, Chien S. Rate sensitivity of shear-induced changes in the lateral diffusion of endothelial cell membrane lipids: a role for membrane perturbation in shear-induced MAPK activation. FASEB J. 2002;16:216–218. - PubMed
-
- Butler PJ, Weinbaum S, Chien S, Lemons DE. Endothelium-dependent, shear-induced vasodilation is rate-sensitive. Microcirculation. 2000;7:53–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

