Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development
- PMID: 17670942
- PMCID: PMC1941790
- DOI: 10.1073/pnas.0703739104
Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development
Abstract
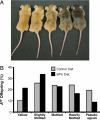
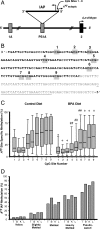
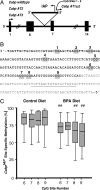
The hypothesis of fetal origins of adult disease posits that early developmental exposures involve epigenetic modifications, such as DNA methylation, that influence adult disease susceptibility. In utero or neonatal exposure to bisphenol A (BPA), a high-production-volume chemical used in the manufacture of polycarbonate plastic, is associated with higher body weight, increased breast and prostate cancer, and altered reproductive function. This study shows that maternal exposure to this endocrine-active compound shifted the coat color distribution of viable yellow agouti (Avy) mouse offspring toward yellow by decreasing CpG (cytosine-guanine dinucleotide) methylation in an intracisternal A particle retrotransposon upstream of the Agouti gene. CpG methylation also was decreased at another metastable locus, the CDK5 activator-binding protein (CabpIAP). DNA methylation at the Avy locus was similar in tissues from the three germ layers, providing evidence that epigenetic patterning during early stem cell development is sensitive to BPA exposure. Moreover, maternal dietary supplementation, with either methyl donors like folic acid or the phytoestrogen genistein, negated the DNA hypomethylating effect of BPA. Thus, we present compelling evidence that early developmental exposure to BPA can change offspring phenotype by stably altering the epigenome, an effect that can be counteracted by maternal dietary supplements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
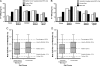
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

