Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer
- PMID: 17670948
- PMCID: PMC1941796
- DOI: 10.1073/pnas.0702387104
Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer
Abstract
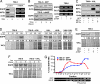
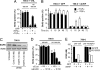
Overexpression of the EGF receptor (EGFR) is a recurrent theme in human cancer and is thought to cause aggressive phenotypes and resistance to standard therapy. There has, thus, been a concerted effort in identifying EGFR gene mutations to explain misregulation of EGFR expression as well as differential sensitivity to anti-EGFR drugs. However, such genetic alterations have proven to be rare occurrences in most types of cancer, suggesting the existence of a more general physiological trigger for aberrant EGFR expression. Here, we provide evidence that overexpression of wild-type EGFR can be induced by the hypoxic microenvironment and activation of hypoxia-inducible factor 2-alpha (HIF2alpha) in the core of solid tumors. Our data suggest that hypoxia/HIF2alpha activation represents a common mechanism for EGFR overexpression by increasing EGFR mRNA translation, thereby diminishing the necessity for gene mutations. This allows for the accumulation of elevated EGFR levels, increasing its availability for the autocrine signaling required for tumor cell growth autonomy. Taken together, our findings provide a nonmutational explanation for EGFR overexpression in human tumors and highlight a role for HIF2alpha activation in the regulation of EGFR protein synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Crit Rev Oncol Hematol. 1995;19:183–232. - PubMed
-
- Yarden Y, Sliwkowski MX. Nat Rev. 2001;2:127–137. - PubMed
-
- Hanahan D, Weinberg RA. Cell. 2000;100:57–70. - PubMed
-
- Arteaga CL. Oncologist. 2002;7:31–39. - PubMed
-
- Mendelsohn J, Baselga J. Oncogene. 2000;19:6550–6565. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

