The GABAA receptor alpha1 subunit epilepsy mutation A322D inhibits transmembrane helix formation and causes proteasomal degradation
- PMID: 17670950
- PMCID: PMC1941799
- DOI: 10.1073/pnas.0700163104
The GABAA receptor alpha1 subunit epilepsy mutation A322D inhibits transmembrane helix formation and causes proteasomal degradation
Abstract
A form of autosomal dominant juvenile myoclonic epilepsy is caused by a nonconservative missense mutation, A322D, in the GABAA receptor alpha1 subunit M3 transmembrane helix. We reported previously that the A322D mutation reduced total and surface alpha1(A322D) subunit protein and that residual alpha1(A322D) subunit resided in the endoplasmic reticulum. Here, we demonstrate that the reduction in alpha1(A322D) expression results from rapid endoplasmic reticulum-associated degradation of the alpha1(A322D) subunit through the ubiquitin-proteasome system. We provide direct evidence that the alpha1(A322D) subunit misfolds and show that in at least 33% of alpha1(A322D) subunits, M3 failed to insert into the lipid bilayer. We constructed a series of mutations in the M3 domain and empirically determined the apparent free energy cost (DeltaGapp) of membrane insertion failure, and we show that the DeltaGapp correlated directly with the recently elucidated transmembrane sequence code (DeltaGLep). These data provide a biochemical mechanism for the pathogenesis of this epilepsy mutation and demonstrate that DeltaGLep predicts the efficiency of lipid partitioning of a naturally occurring protein's transmembrane domain expressed in vivo. Finally, we calculated the DeltaDeltaGLep for 277 known transmembrane missense mutations associated with Charcot-Marie-Tooth disease, diabetes insipidus, retinitis pigmentosa, cystic fibrosis, and severe myoclonic epilepsy of infancy and showed that the majority of these mutations also are likely to destabilize transmembrane domain membrane insertion, but that only a minority of the mutations would be predicted to be as destabilizing as the A322D mutation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

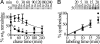

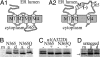

Similar articles
-
Endoplasmic reticulum retention and associated degradation of a GABAA receptor epilepsy mutation that inserts an aspartate in the M3 transmembrane segment of the alpha1 subunit.J Biol Chem. 2005 Nov 11;280(45):37995-8004. doi: 10.1074/jbc.M508305200. Epub 2005 Aug 25. J Biol Chem. 2005. PMID: 16123039
-
GABA(A) receptor alpha1 subunit mutation A322D associated with autosomal dominant juvenile myoclonic epilepsy reduces the expression and alters the composition of wild type GABA(A) receptors.J Biol Chem. 2010 Aug 20;285(34):26390-405. doi: 10.1074/jbc.M110.142299. Epub 2010 Jun 15. J Biol Chem. 2010. PMID: 20551311 Free PMC article.
-
Combining valosin-containing protein (VCP) inhibition and suberanilohydroxamic acid (SAHA) treatment additively enhances the folding, trafficking, and function of epilepsy-associated γ-aminobutyric acid, type A (GABAA) receptors.J Biol Chem. 2015 Jan 2;290(1):325-37. doi: 10.1074/jbc.M114.580324. Epub 2014 Nov 18. J Biol Chem. 2015. PMID: 25406314 Free PMC article.
-
Molecular Pathogenic Basis for GABRG2 Mutations Associated With a Spectrum of Epilepsy Syndromes, From Generalized Absence Epilepsy to Dravet Syndrome.JAMA Neurol. 2016 Aug 1;73(8):1009-16. doi: 10.1001/jamaneurol.2016.0449. JAMA Neurol. 2016. PMID: 27367160 Free PMC article. Review.
-
Mutant GABA(A) receptor subunits in genetic (idiopathic) epilepsy.Prog Brain Res. 2014;213:55-85. doi: 10.1016/B978-0-444-63326-2.00003-X. Prog Brain Res. 2014. PMID: 25194483 Review.
Cited by
-
Genetic Landscape of Common Epilepsies: Advancing towards Precision in Treatment.Int J Mol Sci. 2020 Oct 21;21(20):7784. doi: 10.3390/ijms21207784. Int J Mol Sci. 2020. PMID: 33096746 Free PMC article. Review.
-
Mutations in GABAA receptor subunits associated with genetic epilepsies.J Physiol. 2010 Jun 1;588(Pt 11):1861-9. doi: 10.1113/jphysiol.2010.186999. Epub 2010 Mar 22. J Physiol. 2010. PMID: 20308251 Free PMC article. Review.
-
SAHA (Vorinostat) Corrects Inhibitory Synaptic Deficits Caused by Missense Epilepsy Mutations to the GABAA Receptor γ2 Subunit.Front Mol Neurosci. 2018 Mar 23;11:89. doi: 10.3389/fnmol.2018.00089. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29628874 Free PMC article.
-
Proteostasis regulation of GABAA receptors in neuronal function and disease.Biomed Pharmacother. 2025 May;186:117992. doi: 10.1016/j.biopha.2025.117992. Epub 2025 Mar 20. Biomed Pharmacother. 2025. PMID: 40112516 Free PMC article. Review.
-
Maternal transmission of a rare GABRB3 signal peptide variant is associated with autism.Mol Psychiatry. 2011 Jan;16(1):86-96. doi: 10.1038/mp.2009.118. Epub 2009 Nov 24. Mol Psychiatry. 2011. PMID: 19935738 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

