Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path
- PMID: 17670967
- PMCID: PMC6673074
- DOI: 10.1523/JNEUROSCI.2199-07.2007
Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path
Abstract
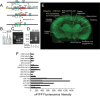
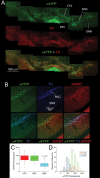
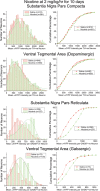
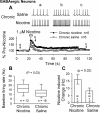
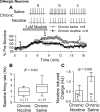
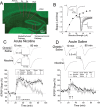
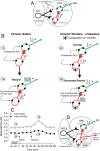
Understanding effects of chronic nicotine requires identifying the neurons and synapses whose responses to nicotine itself, and to endogenous acetylcholine, are altered by continued exposure to the drug. To address this problem, we developed mice whose alpha4 nicotinic receptor subunits are replaced by normally functioning fluorescently tagged subunits, providing quantitative studies of receptor regulation at micrometer resolution. Chronic nicotine increased alpha4 fluorescence in several regions; among these, midbrain and hippocampus were assessed functionally. Although the midbrain dopaminergic system dominates reward pathways, chronic nicotine does not change alpha4* receptor levels in dopaminergic neurons of ventral tegmental area (VTA) or substantia nigra pars compacta. Instead, upregulated, functional alpha4* receptors localize to the GABAergic neurons of the VTA and substantia nigra pars reticulata. In consequence, GABAergic neurons from chronically nicotine-treated mice have a higher basal firing rate and respond more strongly to nicotine; because of the resulting increased inhibition, dopaminergic neurons have lower basal firing and decreased response to nicotine. In hippocampus, chronic exposure to nicotine also increases alpha4* fluorescence on glutamatergic axons of the medial perforant path. In hippocampal slices from chronically treated animals, acute exposure to nicotine during tetanic stimuli enhances induction of long-term potentiation in the medial perforant path, showing that the upregulated alpha4* receptors in this pathway are also functional. The pattern of cell-specific upregulation of functional alpha4* receptors therefore provides a possible explanation for two effects of chronic nicotine: sensitization of synaptic transmission in forebrain and tolerance of dopaminergic neuron firing in midbrain.
Figures

References
-
- Alkondon M, Albuquerque EX. Nicotinic receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional up-regulation by nicotine and to block by bupropion. J Pharmacol Exp Ther. 2005;313:740–750. - PubMed
-
- Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57BL/6J mice. J Neurochem. 2003;84:1431–1441. - PubMed
-
- Cadoni C, Di Chiara G. Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. Eur J Pharmacol. 2000;387:R23–R25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS-11323/NS/NINDS NIH HHS/United States
- R01 DA003194/DA/NIDA NIH HHS/United States
- R01 NS011756/NS/NINDS NIH HHS/United States
- DA-15663/DA/NIDA NIH HHS/United States
- U19 DA019375/DA/NIDA NIH HHS/United States
- R01 DA017279/DA/NIDA NIH HHS/United States
- DA-3194/DA/NIDA NIH HHS/United States
- R21 DA019655/DA/NIDA NIH HHS/United States
- R01 DA009121/DA/NIDA NIH HHS/United States
- NS-11756/NS/NINDS NIH HHS/United States
- DA-19375/DA/NIDA NIH HHS/United States
- R01 NS011323/NS/NINDS NIH HHS/United States
- P30 DA015663/DA/NIDA NIH HHS/United States
- DA-09121/DA/NIDA NIH HHS/United States
- DA-17279/DA/NIDA NIH HHS/United States
- DA-19655/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
