Differential synaptic integration of interneurons in the outer and inner molecular layers of the developing dentate gyrus
- PMID: 17670968
- PMCID: PMC6673061
- DOI: 10.1523/JNEUROSCI.2476-07.2007
Differential synaptic integration of interneurons in the outer and inner molecular layers of the developing dentate gyrus
Abstract
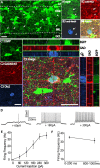
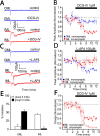
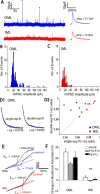
The dentate gyrus (DG) undergoes continued reorganization and lamination during early postnatal development. Interneurons with anatomically identified synaptic contacts migrate from the outer to the inner regions of the molecular layer (ML) of the DG. By using the 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNP)-enhanced green fluorescent protein transgenic mouse, we were able to target and physiologically characterize Dlx2(+) developing ML interneurons. We investigated whether synapses on migrating ML interneurons were functional and defined properties of synaptic inputs onto interneurons that were located in the outer ML (OML) or inner ML (IML). Consistent with ongoing maturation, IML interneurons displayed lower input resistances and more hyperpolarized resting membrane potentials than OML interneurons. Both OML and IML interneurons received a direct excitatory monosynaptic input from the entorhinal cortex via the perforant paths, but this input was differentially sensitive to activation of presynaptic group II and III metabotropic glutamate receptors. Furthermore, only IML interneurons also received significant synaptic input from the CA3/hilar region, especially under conditions of experimentally induced disinhibition. These changes are attributed to a significant reorganization of dendritic fields. GABA(A) receptor-mediated innervation of OML and IML interneurons also displayed significant differences in miniature IPSC amplitude, frequency, and decay kinetics. Finally, cell-attached recordings indicated that GABA(A) receptor activation was depolarizing in OML interneurons but predominantly shunting in IML interneurons. Our data provide evidence that developing ML interneurons receive functional glutamatergic and GABAergic inputs and undergo significant changes in synaptic integration during migration from the OML to the IML.
Figures

Similar articles
-
Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus.J Neurosci. 2008 Jul 23;28(30):7610-23. doi: 10.1523/JNEUROSCI.1355-08.2008. J Neurosci. 2008. PMID: 18650338 Free PMC article.
-
Functional maturation of developing interneurons in the molecular layer of mouse dentate gyrus.Brain Res. 2007 Dec;1186:56-64. doi: 10.1016/j.brainres.2007.09.089. Epub 2007 Oct 12. Brain Res. 2007. PMID: 17996219
-
GABAA currents in immature dentate gyrus granule cells.J Neurophysiol. 1998 Nov;80(5):2255-67. doi: 10.1152/jn.1998.80.5.2255. J Neurophysiol. 1998. PMID: 9819241
-
Synaptic properties of SOM- and CCK-expressing cells in dentate gyrus interneuron networks.J Neurosci. 2014 Jun 11;34(24):8197-209. doi: 10.1523/JNEUROSCI.5433-13.2014. J Neurosci. 2014. PMID: 24920624 Free PMC article.
-
Developmental changes in membrane properties and postsynaptic currents of granule cells in rat dentate gyrus.J Neurophysiol. 1996 Aug;76(2):1074-88. doi: 10.1152/jn.1996.76.2.1074. J Neurophysiol. 1996. PMID: 8871221
Cited by
-
Is plasticity of GABAergic mechanisms relevant to epileptogenesis?Adv Exp Med Biol. 2014;813:133-50. doi: 10.1007/978-94-017-8914-1_11. Adv Exp Med Biol. 2014. PMID: 25012373 Free PMC article. Review.
-
Neurogliaform cells in the molecular layer of the dentate gyrus as feed-forward γ-aminobutyric acidergic modulators of entorhinal-hippocampal interplay.J Comp Neurol. 2011 Jun 1;519(8):1476-91. doi: 10.1002/cne.22577. J Comp Neurol. 2011. PMID: 21452204 Free PMC article.
-
Impact of inhibitory constraint of interneurons on neuronal excitability.J Neurophysiol. 2013 Dec;110(11):2520-35. doi: 10.1152/jn.00047.2013. Epub 2013 Sep 11. J Neurophysiol. 2013. PMID: 24027099 Free PMC article.
-
Synapses on NG2-expressing progenitors in the brain: multiple functions?J Physiol. 2008 Aug 15;586(16):3767-81. doi: 10.1113/jphysiol.2008.158436. Epub 2008 Jul 17. J Physiol. 2008. PMID: 18635642 Free PMC article. Review.
-
Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus.J Neurosci. 2008 Jul 23;28(30):7610-23. doi: 10.1523/JNEUROSCI.1355-08.2008. J Neurosci. 2008. PMID: 18650338 Free PMC article.
References
-
- Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
