Control of chronic pain by the ubiquitin proteasome system in the spinal cord
- PMID: 17670969
- PMCID: PMC6673055
- DOI: 10.1523/JNEUROSCI.5126-06.2007
Control of chronic pain by the ubiquitin proteasome system in the spinal cord
Abstract
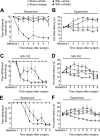
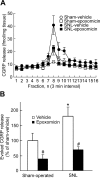
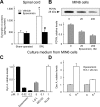
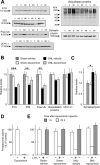
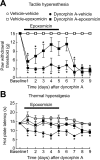
Chronic pain is maintained in part by long-lasting neuroplastic changes in synapses and several proteins critical for synaptic plasticity are degraded by the ubiquitin-proteasome system (UPS). Here, we show that proteasome inhibitors administered intrathecally or subcutaneously prevented the development and reversed nerve injury-induced pain behavior. They also blocked pathological pain induced by sustained administration of morphine or spinal injection of dynorphin A, an endogenous mediator of chronic pain. Proteasome inhibitors blocked mechanical allodynia and thermal hyperalgesia in all three pain models although they did not modify responses to mechanical stimuli, but partially inhibited responses to thermal stimuli in control rats. In the spinal cord, these compounds abolished the enhanced capsaicin-evoked calcitonin gene-related peptide (CGRP) release and dynorphin A upregulation, both elicited by nerve injury. Model experiments demonstrated that the inhibitors may act directly on dynorphin-producing cells, blocking dynorphin secretion. Thus, the effects of proteasome inhibitors on chronic pain were apparently mediated through several cellular mechanisms indispensable for chronic pain, including those of dynorphin A release and postsynaptic actions, and of CGRP secretion. Levels of several UPS proteins were reduced in animals with neuropathic pain, suggesting that UPS downregulation, like effects of proteasome inhibitors, counteracts the development of chronic pain. The inhibitors did not produce marked or disabling motor disturbances at doses that were used to modify chronic pain. These results suggest that the UPS is a critical intracellular regulator of pathological pain, and that UPS-mediated protein degradation is required for maintenance of chronic pain and nociceptive, but not non-nociceptive responses in normal animals.
Figures


References
-
- Adams J, Behnke M, Chen S, Cruickshank AA, Dick LR, Grenier L, Klunder JM, Ma YT, Plamondon L, Stein RL. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg Med Chem Lett. 1998;8:333–338. - PubMed
-
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. - PubMed
-
- Bachar O, Adner M, Uddman R, Cardell LO. Toll-like receptor stimulation induces airway hyper-responsiveness to bradykinin, an effect mediated by JNK and NF-kappa B signaling pathways. Eur J Immunol. 2004;34:1196–1207. - PubMed
-
- Bakalkin G, Yakovleva T, Terenius L. NF-kappa B-like factors in the murine brain. Developmentally regulated and tissue-specific expression. Brain Res Mol Brain Res. 1993;20:137–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
