The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection
- PMID: 17670976
- PMCID: PMC6673047
- DOI: 10.1523/JNEUROSCI.1889-07.2007
The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection
Abstract
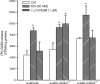
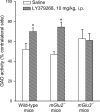
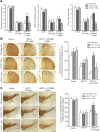
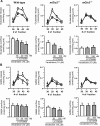
Dual metabotropic glutamate 2/3 (mGlu2/3) receptor agonists have been examined with success in the clinic with positive proof of efficacy in several tests of anxiety and schizophrenia. Moreover, a large body of evidence has accumulated that these drugs have significant neuroprotective potential. An important discussion in the field deals with dissecting effects on mGlu2 versus effects on mGlu3 receptors, which is relevant for the potential use of subtype-selective agonists or allosteric activators. We addressed this issue using mGlu2 and mGlu3 receptor knock-out mice. We used mixed cultures of cortical cells in which astrocytes and neurons were plated at different times and could therefore originate from different mice. Cultures were challenged with NMDA for the induction of excitotoxic neuronal death. The mGlu2/3 receptor agonist, (-)-2-oxa-4-aminocyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY379268), was equally neuroprotective in cultures containing neurons from wild-type, mGlu2-/-, or mGlu3-/- mice. Neuroprotection was instead abolished when astrocytes lacked mGlu3 receptors, unless neuronal mGlu2 receptors were also absent. The latter condition partially restored the protective activity of LY379268. Cultures in which neurons originated from mGlu2-/- mice were also intrinsically resistant to NMDA toxicity. In in vivo experiments, systemic administration of LY379268 protected striatal neurons against NMDA toxicity in wild-type and mGlu2-/- mice but not in mGlu3-/- mice. In addition, LY379268 was protective against nigrostriatal degeneration induced by low doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine only in mice lacking mGlu2 receptors. We conclude that neuroprotection by mGlu2/3 receptor agonists requires the activation of astrocytic mGlu3 receptors, whereas, unexpectedly, activation of mGlu2 receptors might be harmful to neurons exposed to toxic insults.
Figures





References
-
- Ambrosini A, Bresciani L, Fracchia S, Brunello N, Racagni G. Metabotropic glutamate receptors negatively coupled to adenylate cyclase inhibit N-methyl-d-aspartate receptor activity and prevent neurotoxicity in mesencephalic neurons in vitro. Mol Pharmacol. 1995;47:1057–1064. - PubMed
-
- Battaglia G, Monn JA, Schoepp DD. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neurosci Lett. 1997;229:161–164. - PubMed
-
- Battaglia G, Bruno V, Ngomba TR, Di Grezia R, Copani A, Nicoletti F. Selective activation of group-II metabotropic glutamate receptors is protective against excitotoxic neuronal death. Eur J Pharmacol. 1998;356:271–274. - PubMed
-
- Battaglia G, Bruno V, Pisani A, Centonze D, Catania MV, Calabresi P, Nicoletti F. Selective blockade of type-I metabotropic glutamate receptors induces neuroprotection by enhancing GABAergic transmission. Mol Cell Neurosci. 2001;17:1071–1083. - PubMed
-
- Battaglia G, Busceti LC, Pontarelli F, Fornai F, Paparelli A, Bruno V, Ruggieri S, Nicoletti F. Protective role of group-II metabotropic glutamate receptors against nigro-striatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neuropharmacology. 2003;45:155–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
