RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels
- PMID: 17670984
- PMCID: PMC6673065
- DOI: 10.1523/JNEUROSCI.0872-07.2007
RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels
Abstract
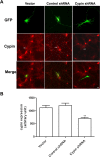
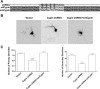
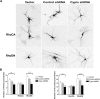
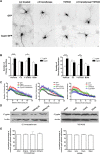
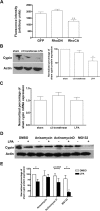
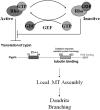
The way a dendrite is patterned determines how a neuron will receive information. The Rho GTPases have been reported to play increasingly well defined roles in determining dendritic branch and spine development and morphology. Much is known about how these small GTPases regulate the actin cytoskeleton; however, very little is known about how they alter the microtubule cytoskeleton. Our laboratory previously cloned and characterized cypin, a guanine deaminase that increases dendrite number by binding to tubulin heterodimers and promoting microtubule assembly. In the present study, we show that cypin and RhoA are part of a common pathway that regulates dendrite number. Inhibition of Rho kinase activity does not affect cypin-mediated dendrite branching. Furthermore, cypin does not affect the activity of RhoA, as measured by GTP binding to RhoA. In fact, activated RhoA acts to inhibit cypin protein expression and, by doing so, decreases dendrite number. In addition, this decrease in cypin protein occurs via a translation-dependent mechanism. Together, our data suggest that cypin acts downstream of the small GTPase RhoA to regulate dendrite branching in hippocampal neurons, providing a novel mechanism for RhoA action on microtubule dynamics.
Figures


References
-
- Ahnert-Hilger G, Holtje M, Grosse G, Pickert G, Mucke C, Nixdorf-Bergweiler B, Boquet P, Hofmann F, Just I. Differential effects of Rho GTPases on axonal and dendritic development in hippocampal neurones. J Neurochem. 2004;90:9–18. - PubMed
-
- Akum BF, Chen M, Gunderson SI, Riefler GM, Scerri-Hansen MM, Firestein BL. Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat Neurosci. 2004;7:145–152. - PubMed
-
- Arimura N, Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48:881–884. - PubMed
-
- Baas PW, Karabay A, Qiang L. Microtubules cut and run. Trends Cell Biol. 2005;15:518–524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
