Top1- and Top2-mediated topological transitions at replication forks ensure fork progression and stability and prevent DNA damage checkpoint activation
- PMID: 17671091
- PMCID: PMC1935030
- DOI: 10.1101/gad.432107
Top1- and Top2-mediated topological transitions at replication forks ensure fork progression and stability and prevent DNA damage checkpoint activation
Abstract
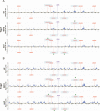
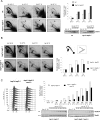
DNA topoisomerases solve topological problems during chromosome metabolism. We investigated where and when Top1 and Top2 are recruited on replicating chromosomes and how their inactivation affects fork integrity and DNA damage checkpoint activation. We show that, in the context of replicating chromatin, Top1 and Top2 act within a 600-base-pair (bp) region spanning the moving forks. Top2 exhibits additional S-phase clusters at specific intergenic loci, mostly containing promoters. TOP1 ablation does not affect fork progression and stability and does not cause activation of the Rad53 checkpoint kinase. top2 mutants accumulate sister chromatid junctions in S phase without affecting fork progression and activate Rad53 at the M-G1 transition. top1 top2 double mutants exhibit fork block and processing and phosphorylation of Rad53 and gamma H2A in S phase. The exonuclease Exo1 influences fork processing and DNA damage checkpoint activation in top1 top2 mutants. Our data are consistent with a coordinated action of Top1 and Top2 in counteracting the accumulation of torsional stress and sister chromatid entanglement at replication forks, thus preventing the diffusion of topological changes along large chromosomal regions. A failure in resolving fork-related topological constrains during S phase may therefore result in abnormal chromosome transitions, DNA damage checkpoint activation, and chromosome breakage during segregation.
Figures





References
-
- Abdurashidova G., Radulescu S., Sandoval O., Zahariev S., Danailov M.B., Demidovich A., Santamaria L., Biamonti G., Riva S., Falaschi A., Radulescu S., Sandoval O., Zahariev S., Danailov M.B., Demidovich A., Santamaria L., Biamonti G., Riva S., Falaschi A., Sandoval O., Zahariev S., Danailov M.B., Demidovich A., Santamaria L., Biamonti G., Riva S., Falaschi A., Zahariev S., Danailov M.B., Demidovich A., Santamaria L., Biamonti G., Riva S., Falaschi A., Danailov M.B., Demidovich A., Santamaria L., Biamonti G., Riva S., Falaschi A., Demidovich A., Santamaria L., Biamonti G., Riva S., Falaschi A., Santamaria L., Biamonti G., Riva S., Falaschi A., Biamonti G., Riva S., Falaschi A., Riva S., Falaschi A., Falaschi A. Functional interactions of DNA topoisomerases with a human replication origin. EMBO J. 2007;26:998–1009. - PMC - PubMed
-
- Alcasabas A.A., Osborn A.J., Bachant J., Hu F., Werler P.J., Bousset K., Furuya K., Diffley J.F., Carr A.M., Elledge S.J., Osborn A.J., Bachant J., Hu F., Werler P.J., Bousset K., Furuya K., Diffley J.F., Carr A.M., Elledge S.J., Bachant J., Hu F., Werler P.J., Bousset K., Furuya K., Diffley J.F., Carr A.M., Elledge S.J., Hu F., Werler P.J., Bousset K., Furuya K., Diffley J.F., Carr A.M., Elledge S.J., Werler P.J., Bousset K., Furuya K., Diffley J.F., Carr A.M., Elledge S.J., Bousset K., Furuya K., Diffley J.F., Carr A.M., Elledge S.J., Furuya K., Diffley J.F., Carr A.M., Elledge S.J., Diffley J.F., Carr A.M., Elledge S.J., Carr A.M., Elledge S.J., Elledge S.J. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat. Cell Biol. 2001;3:958–965. - PubMed
-
- Andrews C.A., Vas A.C., Meier B., Gimenez-Abian J.F., Diaz-Martinez L.A., Green J., Erickson S.L., Vanderwaal K.E., Hsu W.S., Clarke D.J., Vas A.C., Meier B., Gimenez-Abian J.F., Diaz-Martinez L.A., Green J., Erickson S.L., Vanderwaal K.E., Hsu W.S., Clarke D.J., Meier B., Gimenez-Abian J.F., Diaz-Martinez L.A., Green J., Erickson S.L., Vanderwaal K.E., Hsu W.S., Clarke D.J., Gimenez-Abian J.F., Diaz-Martinez L.A., Green J., Erickson S.L., Vanderwaal K.E., Hsu W.S., Clarke D.J., Diaz-Martinez L.A., Green J., Erickson S.L., Vanderwaal K.E., Hsu W.S., Clarke D.J., Green J., Erickson S.L., Vanderwaal K.E., Hsu W.S., Clarke D.J., Erickson S.L., Vanderwaal K.E., Hsu W.S., Clarke D.J., Vanderwaal K.E., Hsu W.S., Clarke D.J., Hsu W.S., Clarke D.J., Clarke D.J. A mitotic topoisomerase II checkpoint in budding yeast is required for genome stability but acts independently of Pds1/securin. Genes & Dev. 2006;20:1162–1174. - PMC - PubMed
-
- Benard M., Maric C., Pierron G., Maric C., Pierron G., Pierron G. DNA replication-dependent formation of joint DNA molecules in Physarum polycephalum. Mol. Cell. 2001;7:971–980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
