The PAS/LOV protein VIVID controls temperature compensation of circadian clock phase and development in Neurospora crassa
- PMID: 17671094
- PMCID: PMC1935033
- DOI: 10.1101/gad.437107
The PAS/LOV protein VIVID controls temperature compensation of circadian clock phase and development in Neurospora crassa
Abstract
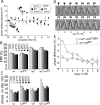
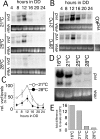
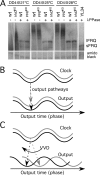
Circadian clocks are cellular timekeepers that regulate aspects of temporal organization on daily and seasonal time scales. To allow accurate time measurement, the period lengths of clocks are conserved in a range of temperatures--a phenomenon known as temperature compensation. Temperature compensation of circadian clock period aids in maintaining a stable "target time" or phase of clock-controlled events. Here we show that the Neurospora protein VIVID (VVD) buffers the circadian system against temperature fluctuations. In vvd-null mutants, the circadian period of clock-controlled events such as asexual sporulation (conidiation) is temperature compensated, but the phase of this clock time marker is not. Consistent with delayed conidiation at lower temperatures in vvd(KO) strains, the levels of vvd gene products in the wild type increase with decreasing temperatures. Moreover, vvd(C108A) mutants that lack the light function of VVD maintain a dark activity that transiently influences the phase of conidiation, indicating that VVD influences the time of conidiation downstream from the clock. FREQUENCY (FRQ) phosphorylation is altered in a vvd(KO) strain, suggesting a mechanism by which VVD can influence the timing of clock-controlled processes in the dark. Thus, temperature compensation of clock-controlled output is a key factor in maintaining temperature compensation of the entire circadian system.
Figures



References
-
- Bell-Pedersen D., Cassone V.M., Earnest D.J., Golden S.S., Hardin P.E., Thomas T.L., Zoran M.J., Cassone V.M., Earnest D.J., Golden S.S., Hardin P.E., Thomas T.L., Zoran M.J., Earnest D.J., Golden S.S., Hardin P.E., Thomas T.L., Zoran M.J., Golden S.S., Hardin P.E., Thomas T.L., Zoran M.J., Hardin P.E., Thomas T.L., Zoran M.J., Thomas T.L., Zoran M.J., Zoran M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005;6:544–556. - PMC - PubMed
-
- Brunner M., Diernfellner A., Diernfellner A. How temperature affects the circadian clock of Neurospora crassa. Chronobiol. Int. 2006;23:81–90. - PubMed
-
- Cheng P., Yang Y.H., Gardner K.H., Liu Y., Yang Y.H., Gardner K.H., Liu Y., Gardner K.H., Liu Y., Liu Y. PAS domain-mediated WC-1/WC-2 interaction is essential for maintaining the steady-state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora. Mol. Cell. Biol. 2002;22:517–524. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
