Intrinsic dynamic behavior of fascin in filopodia
- PMID: 17671164
- PMCID: PMC1995713
- DOI: 10.1091/mbc.e07-04-0346
Intrinsic dynamic behavior of fascin in filopodia
Abstract
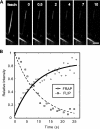
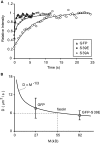
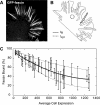
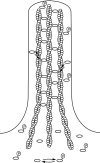
Recent studies showed that the actin cross-linking protein, fascin, undergoes rapid cycling between filopodial filaments. Here, we used an experimental and computational approach to dissect features of fascin exchange and incorporation in filopodia. Using expression of phosphomimetic fascin mutants, we determined that fascin in the phosphorylated state is primarily freely diffusing, whereas actin bundling in filopodia is accomplished by fascin dephosphorylated at serine 39. Fluorescence recovery after photobleaching analysis revealed that fascin rapidly dissociates from filopodial filaments with a kinetic off-rate of 0.12 s(-1) and that it undergoes diffusion at moderate rates with a coefficient of 6 microm(2)s(-1). This kinetic off-rate was recapitulated in vitro, indicating that dynamic behavior is intrinsic to the fascin cross-linker. A computational reaction-diffusion model showed that reversible cross-linking is required for the delivery of fascin to growing filopodial tips at sufficient rates. Analysis of fascin bundling indicated that filopodia are semiordered bundles with one bound fascin per 25-60 actin monomers.
Figures





References
-
- Bryan J., Kane R. E. Separation and interaction of the major components of sea urchin actin gel. J. Mol. Biol. 1978;125:207–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

