When EGF is offside, magnesium is wasted
- PMID: 17671646
- PMCID: PMC1934592
- DOI: 10.1172/JCI33004
When EGF is offside, magnesium is wasted
Abstract
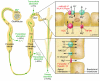
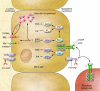
Our understanding of magnesium (Mg(2+)) regulation has recently been catapulted forward by the discovery of several disease loci for monogenic disorders of Mg(2+) homeostasis. In this issue of the JCI, Groenestege et al. report that their study of a rare inherited Mg(2+) wasting disorder in consanguineous kindred shows that EGF acts as an autocrine/paracrine magnesiotropic hormone (see the related article beginning on page 2260). EGF stimulates Mg(2+) reabsorption in the renal distal convoluted tubule (DCT) via engagement of its receptor on the basolateral membrane of DCT cells and activation of the Mg(2+) channel TRPM6 (transient receptor potential cation channel, subfamily M, member 6) in the apical membrane. These authors show that a point mutation in pro-EGF retains EGF secretion to the apical but not the basolateral membrane, disrupting this cascade and causing renal Mg(2+) wasting. This work is another seminal example of the power of the study of monogenic disorders in the quest to understand human physiology.
Figures
Comment on
-
Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia.J Clin Invest. 2007 Aug;117(8):2260-7. doi: 10.1172/JCI31680. J Clin Invest. 2007. PMID: 17671655 Free PMC article. Clinical Trial.
References
-
- Wen S.F., Wong N.L., Dirks J.H. Evidence for renal magnesium secretion during magnesium infusions in the dog. Am. J. Physiol. 1971;220:33–37. - PubMed
-
- Simon D.B., et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. - PubMed
-
- Muller D., Kausalya P.J., Meij I.C., Hunziker W. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: blocking endocytosis restores surface expression of a novel Claudin-16 mutant that lacks the entire C-terminal cytosolic tail. Hum. Mol. Genet. 2006;15:1049–1058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

