Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia
- PMID: 17671655
- PMCID: PMC1934557
- DOI: 10.1172/JCI31680
Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia
Abstract
Primary hypomagnesemia constitutes a rare heterogeneous group of disorders characterized by renal or intestinal magnesium (Mg(2+)) wasting resulting in generally shared symptoms of Mg(2+) depletion, such as tetany and generalized convulsions, and often including associated disturbances in calcium excretion. However, most of the genes involved in the physiology of Mg(2+) handling are unknown. Through the discovery of a mutation in the EGF gene in isolated autosomal recessive renal hypomagnesemia, we have, for what we believe is the first time, identified a magnesiotropic hormone crucial for total body Mg(2+) balance. The mutation leads to impaired basolateral sorting of pro-EGF. As a consequence, the renal EGFR is inadequately stimulated, resulting in insufficient activation of the epithelial Mg(2+) channel TRPM6 (transient receptor potential cation channel, subfamily M, member 6) and thereby Mg(2+) loss. Furthermore, we show that colorectal cancer patients treated with cetuximab, an antagonist of the EGFR, develop hypomagnesemia, emphasizing the significance of EGF in maintaining Mg(2+) balance.
Figures

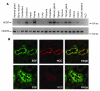
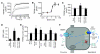
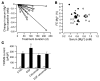
Comment in
-
When EGF is offside, magnesium is wasted.J Clin Invest. 2007 Aug;117(8):2086-9. doi: 10.1172/JCI33004. J Clin Invest. 2007. PMID: 17671646 Free PMC article.
References
-
- Dai L.J., et al. Magnesium transport in the renal distal convoluted tubule. Physiol. Rev. 2001;81:51–84. - PubMed
-
- Ferment O., Garnier P.E., Touitou Y. Comparison of the feedback effect of magnesium and calcium on parathyroid hormone secretion in man. J. Endocrinol. 1987;113:117–122. - PubMed
-
- Cholst I.N., et al. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N. Engl. J. Med. 1984;310:1221–1225. - PubMed
-
- Konrad M., Schlingmann K.P., Gudermann T. Insights into the molecular nature of magnesium homeostasis. Am. J. Physiol. Renal. Physiol. 2004;286:F599–F605. - PubMed
-
- Agus Z.S. Hypomagnesemia. J. Am. Soc. Nephrol. 1999;10:1616–1622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

