Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis
- PMID: 17671656
- PMCID: PMC1934588
- DOI: 10.1172/JCI31947
Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis
Abstract
The inhibition of apoptosis of infected host cells is a well-known but poorly understood function of pathogenic mycobacteria. We show that inactivation of the secA2 gene in Mycobacterium tuberculosis, which encodes a component of a virulence-associated protein secretion system, enhanced the apoptosis of infected macrophages by diminishing secretion of mycobacterial superoxide dismutase. Deletion of secA2 markedly increased priming of antigen-specific CD8(+) T cells in vivo, and vaccination of mice and guinea pigs with a secA2 mutant significantly increased resistance to M. tuberculosis challenge compared with standard M. bovis bacille Calmette-Guérin vaccination. Our results define a mechanism for a key immune evasion strategy of M. tuberculosis and provide what we believe to be a novel approach for improving mycobacterial vaccines.
Figures
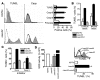
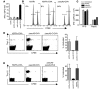
Comment in
-
New TB vaccines: is there a requirement for CD8 T cells?J Clin Invest. 2007 Aug;117(8):2092-4. doi: 10.1172/JCI32933. J Clin Invest. 2007. PMID: 17671648 Free PMC article.
References
-
- Dye C., Watt C.J., Bleed D.M., Hosseini S.M., Raviglione M.C. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–2775. - PubMed
-
- Andersen P., Doherty T.M. The success and failure of BCG — implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005;3:656–662. - PubMed
-
- Kaufmann S.H.E. Introduction. Rational vaccine development against tuberculosis: “Those who don’t remember the past are condemned to repeat it.”. Microbes Infect. 2005;7:897–898. - PubMed
-
- Flynn J.L., Chan J. Immunology of tuberculosis. Annu. Rev. Immun. 2001;19:93–129. - PubMed
-
- Houben E.N., Nguyen L., Pieters J. Interaction of pathogenic mycobacteria with the host immune system. Curr. Opin. Microbiol. 2006;9:76–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

