Retrospective analysis comparing hollow fiber and silicone membrane oxygenators for neonates on ECMO
- PMID: 17672186
- PMCID: PMC4680669
Retrospective analysis comparing hollow fiber and silicone membrane oxygenators for neonates on ECMO
Abstract
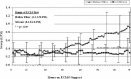
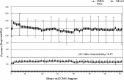
There is little information showing the use of microporous polypropylene hollow fiber oxygenators during extra-corporeal life support (ECLS). Recent surveys have shown increasing use of these hollow fibers amongst ECLS centers in the United States. We performed a retrospective analysis comparing the Terumo BabyRx hollow fiber oxygenator to the Medtronic 800 silicone membrane oxygenator on 14 neonatal patients on extracorporeal membrane oxygenation (ECMO). The aim of this study was to investigate the similarities and differences when comparing pressure drops, prime volumes, oxygenator endurance, and gas transfer capabilities between the two groups.
Conflict of interest statement
The senior author has stated that authors have reported no material, financial or other relationship with any healthcare-related business or other entity whose products or services are discussed in this paper.
Editor’s Note: The BabyRx Hollowfiber oxygenator is approved by the FDA for up to six hours of use. Use beyond six hours is off label.
Figures

Similar articles
-
Development of a new hollow fiber silicone membrane oxygenator for ECMO: the recent progress.Ann Thorac Cardiovasc Surg. 2002 Oct;8(5):268-74. Ann Thorac Cardiovasc Surg. 2002. PMID: 12472408
-
North American neonatal extracorporeal membrane oxygenation (ECMO) devices and team roles: 2008 survey results of Extracorporeal Life Support Organization (ELSO) centers.J Extra Corpor Technol. 2008 Sep;40(3):166-74. J Extra Corpor Technol. 2008. PMID: 18853828 Free PMC article.
-
Silicone-coated polypropylene hollow-fiber oxygenator: experimental evaluation and preliminary clinical use.Ann Thorac Surg. 1997 Jun;63(6):1730-6. doi: 10.1016/s0003-4975(97)00119-7. Ann Thorac Surg. 1997. PMID: 9205175
-
Evolutions of extracorporeal membrane oxygenator (ECMO): perspectives for advanced hollow fiber membrane.J Artif Organs. 2024 Mar;27(1):1-6. doi: 10.1007/s10047-023-01389-w. Epub 2023 Mar 14. J Artif Organs. 2024. PMID: 36914927 Free PMC article. Review.
-
[The development of research on membrane oxgenator application].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2007 Feb;24(1):240-4. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2007. PMID: 17333931 Review. Chinese.
References
-
- Lawson DS, Walczak R, Lawson A, et al. . North American neonatal extracorporeal membrane oxygenation (ECMO) devices: 2002 survey results. J Extra Corpor Technol. 2004;36:16–21. - PubMed
-
- Walczak R, Lawson DS, Kaemmer D, et al. . Evaluation of a preprimed microporous hollow-fiber membrane for rapid response neonatal extracorporeal membrane oxygenation. Perfusion. 2005;20:269–75. - PubMed
-
- Gu YJ, Boonstra PW, Graaff R, et al. . Pressure drop, shear stress, and activation of leukocytes during cardiopulmonary bypass: A comparison between hollow fiber and flat sheet membrane oxygenators. Artif Organs. 2000;24:43–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
