Oxidative stress-induced expression and modulation of Phosphatase of Regenerating Liver-1 (PRL-1) in mammalian retina
- PMID: 17673310
- PMCID: PMC2118714
- DOI: 10.1016/j.bbamcr.2007.06.005
Oxidative stress-induced expression and modulation of Phosphatase of Regenerating Liver-1 (PRL-1) in mammalian retina
Abstract
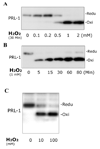
The phosphatase of regenerating liver-1, PRL-1, gene was detected in a screen for foveal cone photoreceptor-associated genes. It encodes a small protein tyrosine phosphatase that was previously immunolocalized to the photoreceptors in primate retina. Here we report that in cones and cone-derived cultured cells both PRL-1 activity and PRL-1 gene expression are modulated under oxidative stress. Oxidation reversibly inhibited the phosphatase activity of PRL-1 due to the formation of an intramolecular disulfide bridge between Cys104 within the active site and another conserved Cys, Cys49. This modulation was observed in vitro, in cell culture and in isolated retinas exposed to hydrogen peroxide. The same treatment caused a rapid increase in PRL-1 expression levels in cultured cells which could be blocked by the protein translation inhibitor, cycloheximide. Increased PRL-1 expression was also observed in living rats subjected to constant light exposure inducing photooxidative stress. We further demonstrated that both oxidation and overexpression of PRL-1 upon oxidative stress are greatly enhanced by inhibition of the glutathione system responsible for cellular redox regulation. These findings suggest that PRL-1 is a molecular component of the photoreceptor's response to oxidative stress acting upstream of the glutathione system.
Figures







Similar articles
-
Expression of the protein tyrosine phosphatase, phosphatase of regenerating liver 1, in the outer segments of primate cone photoreceptors.Brain Res Mol Brain Res. 2000 Apr 14;77(1):95-103. doi: 10.1016/s0169-328x(00)00045-0. Brain Res Mol Brain Res. 2000. PMID: 10814835
-
Enzyme activity of phosphatase of regenerating liver is controlled by the redox environment and its C-terminal residues.Biochemistry. 2009 May 26;48(20):4262-72. doi: 10.1021/bi900241k. Biochemistry. 2009. PMID: 19341304 Free PMC article.
-
Thioredoxin-related protein 32 (TRP32) specifically reduces oxidized phosphatase of regenerating liver (PRL).J Biol Chem. 2013 Mar 8;288(10):7263-70. doi: 10.1074/jbc.M112.418004. Epub 2013 Jan 28. J Biol Chem. 2013. PMID: 23362275 Free PMC article.
-
Molecular mechanisms of the PRL phosphatases.FEBS J. 2013 Jan;280(2):505-24. doi: 10.1111/j.1742-4658.2012.08565.x. Epub 2012 Apr 10. FEBS J. 2013. PMID: 22413991 Review.
-
PRL-3: a metastasis-associated phosphatase in search of a function.Cells Tissues Organs. 2007;185(1-3):232-6. doi: 10.1159/000101324. Cells Tissues Organs. 2007. PMID: 17587829 Review.
Cited by
-
PRL2 Controls Phagocyte Bactericidal Activity by Sensing and Regulating ROS.Front Immunol. 2018 Nov 13;9:2609. doi: 10.3389/fimmu.2018.02609. eCollection 2018. Front Immunol. 2018. PMID: 30483267 Free PMC article.
-
Suppressing endoplasmic reticulum stress-related autophagy attenuates retinal light injury.Aging (Albany NY). 2020 Aug 28;12(16):16579-16596. doi: 10.18632/aging.103846. Epub 2020 Aug 28. Aging (Albany NY). 2020. PMID: 32858529 Free PMC article.
-
Critical Roles of Dual-Specificity Phosphatases in Neuronal Proteostasis and Neurological Diseases.Int J Mol Sci. 2017 Sep 13;18(9):1963. doi: 10.3390/ijms18091963. Int J Mol Sci. 2017. PMID: 28902166 Free PMC article. Review.
-
Hydrogen Sulfide: Novel Endogenous and Exogenous Modulator of Oxidative Stress in Retinal Degeneration Diseases.Molecules. 2021 Apr 21;26(9):2411. doi: 10.3390/molecules26092411. Molecules. 2021. PMID: 33919146 Free PMC article. Review.
-
Functionally enhanced placenta-derived mesenchymal stem cells inhibit adipogenesis in orbital fibroblasts with Graves' ophthalmopathy.Stem Cell Res Ther. 2020 Nov 5;11(1):469. doi: 10.1186/s13287-020-01982-3. Stem Cell Res Ther. 2020. PMID: 33153489 Free PMC article.
References
-
- Yarovinsky TO, Rickman DW, Diamond RH, Taub R, Hageman GS, Rickman C Bowes. Expression of the protein tyrosine phosphatase, phosphatase of regenerating liver 1, in the outer segments of primate cone photoreceptors. Brain Res Mol Brain Res. 2000;77:95–103. - PubMed
-
- Peng Y, Genin A, Spinner NB, Diamond RH, Taub R. The gene encoding human nuclear protein tyrosine phosphatase, PRL-1. Cloning, chromosomal localization, and identification of an intron enhancer. J Biol Chem. 1998;273:17286–17295. - PubMed
-
- Gehrig A, Felbor U, Kelsell RE, Hunt DM, Maumenee IH, Weber BH. Assessment of the interphotoreceptor matrix proteoglycan-1 (IMPG1) gene localised to 6q13-q15 in autosomal dominant Stargardt-like disease (ADSTGD), progressive bifocal chorioretinal atrophy (PBCRA), and North Carolina macular dystrophy (MCDR1) J Med Genet. 1998;35:641–645. - PMC - PubMed
-
- Zeng Q, Hong W, Tan YH. Mouse PRL-2 and PRL-3, two potentially prenylated protein tyrosine phosphatases homologous to PRL-1. Biochem Biophys Res Commun. 1998;244:421–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

