TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA
- PMID: 17673906
- PMCID: PMC1994119
- DOI: 10.1038/sj.emboj.7601818
TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA
Abstract
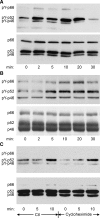
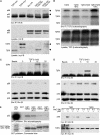
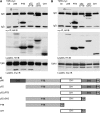
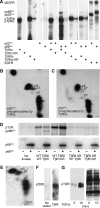
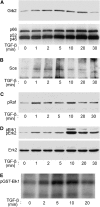
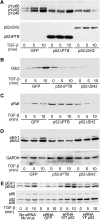
Erk1/Erk2 MAP kinases are key regulators of cell behaviour and their activation is generally associated with tyrosine kinase signalling. However, TGF-beta stimulation also activates Erk MAP kinases through an undefined mechanism, albeit to a much lower level than receptor tyrosine kinase stimulation. We report that upon TGF-beta stimulation, the activated TGF-beta type I receptor (TbetaRI) recruits and directly phosphorylates ShcA proteins on tyrosine and serine. This dual phosphorylation results from an intrinsic TbetaRI tyrosine kinase activity that complements its well-defined serine-threonine kinase function. TGF-beta-induced ShcA phosphorylation induces ShcA association with Grb2 and Sos, thereby initiating the well-characterised pathway linking receptor tyrosine kinases with Erk MAP kinases. We also found that TbetaRI is tyrosine phosphorylated in response to TGF-beta. Thus, TbetaRI, like the TGF-beta type II receptor, is a dual-specificity kinase. Recruitment of tyrosine kinase signalling pathways may account for aspects of TGF-beta biology that are independent of Smad signalling.
Figures


References
-
- Attisano L, Wrana JL (2002) Signal transduction by the TGF-β superfamily. Science 296: 1646–1647 - PubMed
-
- Cano E, Hazzalin CA, Kardalinou E, Buckle RS, Mahadevan LC (1995) Neither ERK nor JNK/SAPK MAP kinase subtypes are essential for histone H3/HMG-14 phosphorylation or c-fos and c-jun induction. J Cell Sci 108: 3599–3609 - PubMed
-
- Chen RH, Moses HL, Maruoka EM, Derynck R, Kawabata M (1995) Phosphorylation-dependent interaction of the cytoplasmic domains of the type I and type II transforming growth factor-β receptors. J Biol Chem 270: 12235–12241 - PubMed
-
- Cooper JA, Sefton BM, Hunter T (1983) Detection and quantification of phosphotyrosine in proteins. Methods Enzymol 99: 387–402 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

