Fluorescence depolarization studies of filamentous actin analyzed with a genetic algorithm
- PMID: 17675344
- PMCID: PMC2025662
- DOI: 10.1529/biophysj.107.107920
Fluorescence depolarization studies of filamentous actin analyzed with a genetic algorithm
Abstract
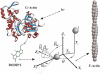
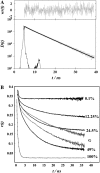
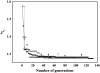
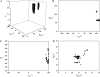
A new method, in which a genetic algorithm was combined with Brownian dynamics and Monte Carlo simulations, was developed to analyze fluorescence depolarization data collected by the time-correlated single photon-counting technique. It was applied to studies of BODIPY-labeled filamentous actin (F-actin). The technique registered the local order and reorienting motions of the fluorophores, which were covalently coupled to cysteine 374 (C374) in actin and interacted by electronic energy migration within the actin polymers. Analyses of F-actin samples composed of different fractions of labeled actin molecules revealed the known helical organization of F-actin, demonstrating the usefulness of this technique for structure determination of complex protein polymers. The distance from the filament axis to the fluorophore was found to be considerably less than expected from the proposed position of C374 at a high filament radius. In addition, polymerization experiments with BODIPY-actin suggest a 25-fold more efficient signal for filament formation than pyrene-actin.
Figures

References
-
- Dickerson, R. E. 1964. X-Ray Analysis and Protein Structure. Academic Press, New York.
-
- Blundell, T. L., and L. N. Johnson. 1976. Protein Crystallography. Academic Press, London.
-
- Clore, G. M., and A. M. Gronenborn. 1993. Topics in Molecular and Structural Biology. The Macmillan Press, London.
-
- Wütrich, K. 1986. NMR of Proteins and Nucleic Acids. Wiley, New York.
-
- Van der Meer, B. W., G. Coker III, and S.-Y. S. Chen. 1994. Resonance Energy Transfer: Theory and Data. VCH Publishers, New York.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

