Triton X-100 partitioning into sphingomyelin bilayers at subsolubilizing detergent concentrations: effect of lipid phase and a comparison with dipalmitoylphosphatidylcholine
- PMID: 17675347
- PMCID: PMC2072071
- DOI: 10.1529/biophysj.107.104463
Triton X-100 partitioning into sphingomyelin bilayers at subsolubilizing detergent concentrations: effect of lipid phase and a comparison with dipalmitoylphosphatidylcholine
Abstract
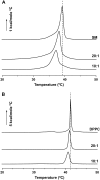
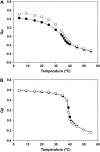
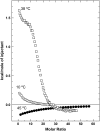
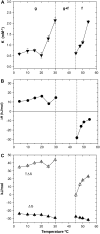
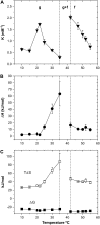
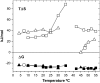
We examined the partitioning of the nonionic detergent Triton X-100 at subsolubilizing concentrations into bilayers of either egg sphingomyelin (SM), palmitoyl SM, or dipalmitoylphosphatidylcholine. SM is known to require less detergent than phosphatidylcholine to achieve the same extent of solubilization, and for all three phospholipids solubilization is temperature dependent. In addition, the three lipids exhibit a gel-fluid phase transition in the 38-41 degrees C temperature range. Experiments have been performed at Triton X-100 concentrations well below the critical micellar concentration, so that only detergent monomers have to be considered. Lipid/detergent mol ratios were never <10:1, thus ensuring that the solubilization stage was never reached. Isothermal titration calorimetry, DSC, and infrared, fluorescence, and (31)P-NMR spectroscopies were applied in the 5-55 degrees C temperature range. The results show that, irrespective of the chemical nature of the lipid, DeltaG degrees of partitioning remained in the range of -27 kJ/mol lipid in the gel phase and of -30 kJ/mol lipid in the fluid phase. This small difference cannot account for the observed phase-dependent differences in solubilization. Such virtually constant DeltaG degrees occurred as a result of the compensation of enthalpic and entropic components, which varied with both temperature and lipid composition. Consequently, the observed different susceptibilities to solubilization cannot be attributed to differential binding but to further events in the solubilization process, e.g., bilayer saturability by detergent or propensity to form lipid-detergent mixed micelles. The data here shed light on the relatively unexplored early stages of membrane solubilization and open new ways to understand the phenomenon of membrane resistance toward detergent solubilization.
Figures
References
-
- Tanford, P. 1973. The Hydrophobic Effect. Wiley, New York.
-
- Helenius, A., and K. Simons. 1975. Solubilization of membranes by detergents. Biochim. Biophys. Acta. 415:29–79. - PubMed
-
- Goni, F. M., and A. Alonso. 2000. Detergents in biomembrane studies. Biochim. Biophys. Acta. 1508:1–256. - PubMed
-
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. - PubMed
-
- Lichtenberg, D., F. M. Goni, and H. Heerklotz. 2005. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 30:430–436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

