Biofilm interactions between distinct bacterial genera isolated from drinking water
- PMID: 17675433
- PMCID: PMC2075010
- DOI: 10.1128/AEM.00837-07
Biofilm interactions between distinct bacterial genera isolated from drinking water
Abstract
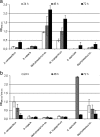
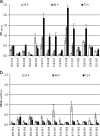
In the environment, multiple microorganisms coexist as communities, competing for resources and often associated as biofilms. In this study, single- and dual-species biofilm formation by, and specific activities of, six heterotrophic intergeneric bacteria were determined using 96-well polystyrene plates over a 72-h period. These bacteria were isolated from drinking water and identified by partial 16S rRNA gene sequencing. A series of planktonic studies was also performed, assessing the bacterial growth rate, motility, and production of quorum-sensing inhibitors (QSI). This constituted an attempt to identify key attributes allowing bacteria to effectively interact and coexist in a drinking-water environment. We observed that in both pure and dual cultures, all of the isolates formed stable biofilms within 72 h, with specific metabolic activity decreasing, in most cases, with an increase in biofilm mass. The largest single- and dual-biofilm amounts were found for Methylobacterium sp. and the combination of Methylobacterium sp. and Mycobacterium mucogenicum, respectively. Evidences of microbial interactions in dual-biofilm formation, associated with appreciable biomass variation in comparison with single biofilms, were found for the following cases: synergy/cooperation between Sphingomonas capsulata and Burkholderia cepacia, S. capsulata and Staphylococcus sp., and B. cepacia and Acinetobacter calcoaceticus and antagonism between S. capsulata and M. mucogenicum, S. capsulata and A. calcoaceticus, and M. mucogenicum and Staphylococcus sp. A neutral interaction was found for Methylobacterium sp.-M. mucogenicum, S. capsulata-Staphylococcus sp., M. mucogenicum-A. calcoaceticus, and Methylobacterium sp.-A. calcoaceticus biofilms, since the resultant dual biofilms had a mass and specific metabolic activity similar to the average for each single biofilm. B. cepacia had the highest growth rate and motility and produced QSI. Other bacteria producing QSI were Methylobacterium sp., S. capsulata, and Staphylococcus sp. However, only for S. capsulata-M. mucogenicum, S. capsulata-A. calcoaceticus, and M. mucogenicum-Staphylococcus sp., dual-biofilm formation seems to be regulated by the QSI produced by S. capsulata and Staphylococcus sp. and by the increased growth rate of S. capsulata. The parameters assessed by planktonic studies did not allow prediction and generalization of the exact mechanism regulating dual-species biofilm formation between the drinking-water bacteria.
Figures
Similar articles
-
Adhesion and biofilm formation on polystyrene by drinking water-isolated bacteria.Antonie Van Leeuwenhoek. 2010 Oct;98(3):317-29. doi: 10.1007/s10482-010-9444-2. Epub 2010 Apr 20. Antonie Van Leeuwenhoek. 2010. PMID: 20405208
-
The effects of metabolite molecules produced by drinking water-isolated bacteria on their single and multispecies biofilms.Biofouling. 2011 Aug;27(7):685-99. doi: 10.1080/08927014.2011.597502. Biofouling. 2011. PMID: 21732713
-
Intergeneric coaggregation among drinking water bacteria: evidence of a role for Acinetobacter calcoaceticus as a bridging bacterium.Appl Environ Microbiol. 2008 Feb;74(4):1259-63. doi: 10.1128/AEM.01747-07. Epub 2007 Dec 21. Appl Environ Microbiol. 2008. PMID: 18156333 Free PMC article.
-
Bacterial interactions in dental biofilm development.J Dent Res. 2009 Nov;88(11):982-90. doi: 10.1177/0022034509346811. J Dent Res. 2009. PMID: 19828884 Review.
-
Quorum sensing in biofilms--how to destroy the bacterial citadels or their cohesion/power?Anaerobe. 2011 Dec;17(6):280-5. doi: 10.1016/j.anaerobe.2011.03.023. Epub 2011 Apr 8. Anaerobe. 2011. PMID: 21497662 Review.
Cited by
-
Understanding the Interactions between Staphylococcus aureus and the Raw-Meat-Processing Environment Isolate Klebsiella oxytoca in Dual-Species Biofilms via Discovering an Altered Metabolic Profile.Microorganisms. 2021 Mar 24;9(4):672. doi: 10.3390/microorganisms9040672. Microorganisms. 2021. PMID: 33805148 Free PMC article.
-
Mitigation and use of biofilms in space for the benefit of human space exploration.Biofilm. 2023 Jan 6;5:100102. doi: 10.1016/j.bioflm.2022.100102. eCollection 2023 Dec. Biofilm. 2023. PMID: 36660363 Free PMC article.
-
Comparative genomics of the transportome of Ten Treponema species.Microb Pathog. 2019 Jul;132:87-99. doi: 10.1016/j.micpath.2019.04.034. Epub 2019 Apr 25. Microb Pathog. 2019. PMID: 31029716 Free PMC article.
-
New developments in microbial interspecies signaling.Curr Opin Microbiol. 2009 Apr;12(2):205-14. doi: 10.1016/j.mib.2009.01.003. Epub 2009 Feb 27. Curr Opin Microbiol. 2009. PMID: 19251475 Free PMC article. Review.
-
Highly efficient phenol degradation in a batch moving bed biofilm reactor: benefiting from biofilm-enhancing bacteria.World J Microbiol Biotechnol. 2018 Oct 28;34(11):164. doi: 10.1007/s11274-018-2543-3. World J Microbiol Biotechnol. 2018. PMID: 30368594
References
-
- Berry, D., C. Xi, and L. Raskin. 2006. Microbiol ecology of drinking water distribution systems. Curr. Opin. Microbiol. 17:297-302. - PubMed
-
- Bhattarai, H. D., Y. K. Lee, K. H. Cho, H. K. Lee, and H. W. Shin. 2006. The study of antagonistic interactions among pelagic bacteria: a promising way to coin environmentally friendly antifouling compounds. Hydrobiologia 568:417-423.
-
- Block, J. C. 1992. Biofilms in drinking water distribution systems, p. 469-485. In T. R. Bott, L. Melo, M. Fletcher, and B. Capedeville (ed.), Biofilms—science and technology. Kluwer, Dordrecht, The Netherlands.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

