Novel sensitive high-throughput screening strategy for nitrilase-producing strains
- PMID: 17675436
- PMCID: PMC2075032
- DOI: 10.1128/AEM.01089-07
Novel sensitive high-throughput screening strategy for nitrilase-producing strains
Abstract
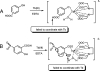
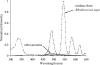
Nitrilases have found wide use in the pharmaceutical industry for the production of fine chemicals, and it is important to have a method by which to screen libraries of isolated or engineered nitrilase variants (including bacteria and fungi). The conventional methods, such as high-performance liquid chromatography, liquid chromatography-mass spectrometry, capillary electrophoresis, or gas chromatography, are tedious and time-consuming. Therefore, a direct and sensitive readout of the nitrilase's activity has to be considered. In this paper, we report a novel time-resolved luminescent probe: o-hydroxybenzonitrile derivatives could be applied to detect the activity of the nitrilases. By the action of nitrilases, o-hydroxybenzonitrile derivatives can be transformed to the corresponding salicylic acid derivatives, which, upon binding Tb(3+), serve as a photon antenna and sensitize Tb(3+) luminescence. Because of the time-resolved property of the luminescence, the background from the other proteins (especially in the fermentation system) in the assay could be reduced and, therefore, the sensitivity was increased. Moreover, because the detection was performed on a 96- or 384-well plate, the activity of the nitrilases from microorganisms could be determined quickly. Based on this strategy, the best fermentation conditions for nitrilase-producing strains were obtained.
Figures




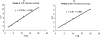

Similar articles
-
Nitrilases in nitrile biocatalysis: recent progress and forthcoming research.Microb Cell Fact. 2012 Oct 30;11:142. doi: 10.1186/1475-2859-11-142. Microb Cell Fact. 2012. PMID: 23106943 Free PMC article. Review.
-
Nitrilase enzymes and their role in plant-microbe interactions.Microb Biotechnol. 2009 Jul;2(4):441-51. doi: 10.1111/j.1751-7915.2009.00111.x. Epub 2009 Apr 16. Microb Biotechnol. 2009. PMID: 21255276 Free PMC article. Review.
-
Recent advances and challenges in the heterologous production of microbial nitrilases for biocatalytic applications.World J Microbiol Biotechnol. 2017 Jan;33(1):8. doi: 10.1007/s11274-016-2173-6. Epub 2016 Nov 17. World J Microbiol Biotechnol. 2017. PMID: 27858339 Review.
-
Biotransformation of heterocyclic dinitriles by Rhodococcus erythropolis and fungal nitrilases.Biotechnol Lett. 2007 Jul;29(7):1119-24. doi: 10.1007/s10529-007-9364-z. Epub 2007 May 4. Biotechnol Lett. 2007. PMID: 17479225
-
A nitrilase from a metagenomic library acts regioselectively on aliphatic dinitriles.Appl Microbiol Biotechnol. 2011 Jan;89(1):91-8. doi: 10.1007/s00253-010-2831-9. Epub 2010 Aug 20. Appl Microbiol Biotechnol. 2011. PMID: 20725724
Cited by
-
Nitrilases in nitrile biocatalysis: recent progress and forthcoming research.Microb Cell Fact. 2012 Oct 30;11:142. doi: 10.1186/1475-2859-11-142. Microb Cell Fact. 2012. PMID: 23106943 Free PMC article. Review.
-
From sequence to function: a new workflow for nitrilase identification.Appl Microbiol Biotechnol. 2020 Jun;104(11):4957-4970. doi: 10.1007/s00253-020-10544-9. Epub 2020 Apr 14. Appl Microbiol Biotechnol. 2020. PMID: 32291488 Free PMC article.
References
-
- Banerjee, A., R. Sharma, and U. C. Banerjee. 2002. The nitrile-degrading enzymes: current status and future prospects. Appl. Microbiol. Biotechnol. 60:33-44. - PubMed
-
- Barrios, A. M., and C. S. Craik. 2002. Scanning the prime-site substrate 3 specificity of proteolytic enzymes: a novel assay based on ligand-enhanced lanthanide ion fluorescence. Bioorg. Med. Chem. Lett. 12:3619-3623. - PubMed
-
- Bryden, C. C., and C. N. Reilley. 1987. Europium luminescence lifetimes and spectra for evaluation of 11 europium complexes as aqueous shift reagents for nuclear magnetic resonance spectrometry. Anal. Chem. 54:610-615.
-
- Chen, G., D. J. Yee, N. G. Gubernator, and D. Sames 2005. Design of optical switches as metabolic indicators: new fluorogenic probes for monoamine oxidase (MAO A and B). J. Am. Chem. Soc. 127:4544-4545. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

