Phospholipase cbeta is critical for T cell chemotaxis
- PMID: 17675482
- PMCID: PMC3228861
- DOI: 10.4049/jimmunol.179.4.2223
Phospholipase cbeta is critical for T cell chemotaxis
Abstract
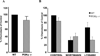
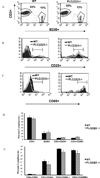
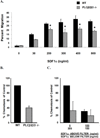
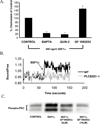
Chemokines acting through G protein-coupled receptors play an essential role in the immune response. PI3K and phospholipase C (PLC) are distinct signaling molecules that have been proposed in the regulation of chemokine-mediated cell migration. Studies with knockout mice have demonstrated a critical role for PI3K in G(alphai) protein-coupled receptor-mediated neutrophil and lymphocyte chemotaxis. Although PLCbeta is not essential for the chemotactic response of neutrophils, its role in lymphocyte migration has not been clearly defined. We compared the chemotactic response of peripheral T cells derived from wild-type mice with mice containing loss-of-function mutations in both of the two predominant lymphocyte PLCbeta isoforms (PLCbeta2 and PLCbeta3), and demonstrate that loss of PLCbeta2 and PLCbeta3 significantly impaired T cell migration. Because second messengers generated by PLCbeta lead to a rise in intracellular calcium and activation of PKC, we analyzed which of these responses was critical for the PLCbeta-mediated chemotaxis. Intracellular calcium chelation decreased the chemotactic response of wild-type lymphocytes, but pharmacologic inhibition of several PKC isoforms had no effect. Furthermore, calcium efflux induced by stromal cell-derived factor-1alpha was undetectable in PLCbeta2beta3-null lymphocytes, suggesting that the migration defect is due to the impaired ability to increase intracellular calcium. This study demonstrates that, in contrast to neutrophils, phospholipid second messengers generated by PLCbeta play a critical role in T lymphocyte chemotaxis.
Figures



References
-
- Cyster J. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;Vol. 286:2098. - PubMed
-
- Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;Vol. 2:108. - PubMed
-
- Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;Vol. 2:123. - PubMed
-
- Loetscher P, Moser B, Baggiolini M. Chemokines and their receptors in lymphocyte traffic and HIV infection. Adv Immunol. 2000;Vol. 74:127. - PubMed
-
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;Vol. 12:121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

