IFN-gamma-producing dendritic cells are important for priming of gut intraepithelial lymphocyte response against intracellular parasitic infection
- PMID: 17675510
- PMCID: PMC3109618
- DOI: 10.4049/jimmunol.179.4.2485
IFN-gamma-producing dendritic cells are important for priming of gut intraepithelial lymphocyte response against intracellular parasitic infection
Abstract
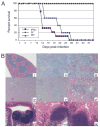
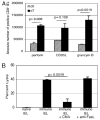
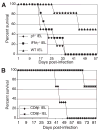
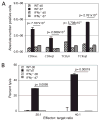
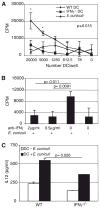
The importance of intraepithelial lymphocytes (IEL) in immunoprotection against orally acquired pathogens is being increasingly recognized. Recent studies have demonstrated that Ag-specific IEL can be generated and can provide an important first line of defense against pathogens acquired via oral route. However, the mechanism involved in priming of IEL remains elusive. Our current study, using a microsporidial model of infection, demonstrates that priming of IEL is dependent on IFN-gamma-producing dendritic cells (DC) from mucosal sites. DC from mice lacking the IFN-gamma gene are unable to prime IEL, resulting in failure of these cells to proliferate and lyse pathogen-infected targets. Also, treatment of wild-type DC from Peyer's patches with Ab to IFN-gamma abrogates their ability to prime an IEL response against Encephalitozoon cuniculi in vitro. Moreover, when incubated with activated DC from IFN-gamma knockout mice, splenic CD8(+) T cells are not primed efficiently and exhibit reduced ability to home to the gut compartment. These data strongly suggest that IFN-gamma-producing DC from mucosal sites play an important role in the generation of an Ag-specific IEL response in the small intestine. To our knowledge, this report is the first demonstrating a role for IFN-gamma-producing DC from Peyer's patches in the development of Ag-specific IEL population and their trafficking to the gut epithelium.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures
Similar articles
-
Interleukin-12-producing CD103+ CD11b- CD8+ dendritic cells are responsible for eliciting gut intraepithelial lymphocyte response against Encephalitozoon cuniculi.Infect Immun. 2015 Dec;83(12):4719-30. doi: 10.1128/IAI.00820-15. Epub 2015 Sep 28. Infect Immun. 2015. PMID: 26416905 Free PMC article.
-
Induction of a rapid and strong antigen-specific intraepithelial lymphocyte response during oral Encephalitozoon cuniculi infection.J Immunol. 2004 Apr 1;172(7):4402-9. doi: 10.4049/jimmunol.172.7.4402. J Immunol. 2004. PMID: 15034055 Free PMC article.
-
Gamma delta T cell-deficient mice have a down-regulated CD8+ T cell immune response against Encephalitozoon cuniculi infection.J Immunol. 2001 Jun 15;166(12):7389-97. doi: 10.4049/jimmunol.166.12.7389. J Immunol. 2001. PMID: 11390490
-
Dendritic Cell Subsets in Intestinal Immunity and Inflammation.J Immunol. 2020 Mar 1;204(5):1075-1083. doi: 10.4049/jimmunol.1900710. J Immunol. 2020. PMID: 32071090 Review.
-
Peyer's patch dendritic cells as regulators of mucosal adaptive immunity.Cell Mol Life Sci. 2005 Jun;62(12):1333-8. doi: 10.1007/s00018-005-5037-z. Cell Mol Life Sci. 2005. PMID: 15971108 Free PMC article. Review.
Cited by
-
The state of research for AIDS-associated opportunistic infections and the importance of sustaining smaller research communities.Eukaryot Cell. 2012 Feb;11(2):90-7. doi: 10.1128/EC.05143-11. Epub 2011 Dec 9. Eukaryot Cell. 2012. PMID: 22158712 Free PMC article. No abstract available.
-
Production of IFN-γ by splenic dendritic cells during innate immune responses against Francisella tularensis LVS depends on MyD88, but not TLR2, TLR4, or TLR9.PLoS One. 2020 Aug 3;15(8):e0237034. doi: 10.1371/journal.pone.0237034. eCollection 2020. PLoS One. 2020. PMID: 32745117 Free PMC article.
-
Autocrine, not paracrine, interferon-gamma gene delivery enhances ex vivo antigen-specific cytotoxic T lymphocyte stimulation and killing.J Biomed Biotechnol. 2010;2010:270985. doi: 10.1155/2010/270985. Epub 2010 May 13. J Biomed Biotechnol. 2010. PMID: 20490265 Free PMC article.
-
Phagocytosis Is the Sole Arm of Drosophila melanogaster Known Host Defenses That Provides Some Protection Against Microsporidia Infection.Front Immunol. 2022 Apr 13;13:858360. doi: 10.3389/fimmu.2022.858360. eCollection 2022. Front Immunol. 2022. PMID: 35493511 Free PMC article.
-
NK cells in mucosal defense against infection.Biomed Res Int. 2014;2014:413982. doi: 10.1155/2014/413982. Epub 2014 Aug 14. Biomed Res Int. 2014. PMID: 25197644 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

