gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion
- PMID: 17675580
- PMCID: PMC1959478
- DOI: 10.2353/ajpath.2007.070008
gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion
Abstract
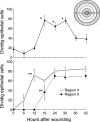
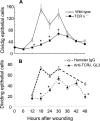
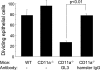
Corneal epithelial abrasion in C57BL/6 mice induces an inflammatory response with peak accumulation of neutrophils in the corneal stroma within 12 hours. Platelets localize in the limbal vessels throughout the same time course as neutrophils and contribute to wound healing because antibody-dependent depletion of platelets retards epithelial division and wound closure. In the present study, T cells in the limbal epithelium were found to predominantly express the gammadelta T-cell receptor (TCR). Corneal abrasion in wild-type, CD11a(-/-), and P-sel(-/-) mice increased the numbers of gammadelta T cells in the limbal and peripheral corneal epithelium and in the corneal stroma adjacent to the limbal blood vessels. Intercellular adhesion molecule (ICAM)-1(-/-) mice exhibited a reduction in gammadelta T-cell accumulation. TCRdelta(-/-) mice exhibited reduced inflammation and delayed epithelial wound healing as evidenced by delayed wound closure, reduced epithelial cell division, reduced neutrophil infiltration, and reduced epithelial cell density at 96 hours after wounding. TCRdelta(-/-) mice also exhibited >60% reduction in platelet localization in the limbus despite similar platelet counts and platelet function assessed with an in vivo thrombosis model. These results are consistent with the conclusion that gammadelta T cells are necessary for efficient inflammation, platelet localization in the limbus, and epithelial wound healing after corneal abrasion.
Figures






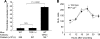
Similar articles
-
CCL20, γδ T cells, and IL-22 in corneal epithelial healing.FASEB J. 2011 Aug;25(8):2659-68. doi: 10.1096/fj.11-184804. Epub 2011 Apr 25. FASEB J. 2011. PMID: 21518851 Free PMC article.
-
Platelet response to corneal abrasion is necessary for acute inflammation and efficient re-epithelialization.Invest Ophthalmol Vis Sci. 2006 Nov;47(11):4794-802. doi: 10.1167/iovs.06-0381. Invest Ophthalmol Vis Sci. 2006. PMID: 17065490
-
ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing.Am J Pathol. 2009 Aug;175(2):571-9. doi: 10.2353/ajpath.2009.090112. Epub 2009 Jul 16. Am J Pathol. 2009. PMID: 19608878 Free PMC article.
-
Lymphocyte function-associated antigen-1-dependent inhibition of corneal wound healing.Am J Pathol. 2006 Nov;169(5):1590-600. doi: 10.2353/ajpath.2006.060415. Am J Pathol. 2006. PMID: 17071583 Free PMC article.
-
[Wound healing following amniotic membrane, limbal stem cell and corneal transplantation].Ophthalmologe. 2020 Dec;117(12):1163-1170. doi: 10.1007/s00347-020-01211-5. Ophthalmologe. 2020. PMID: 32833114 Review. German.
Cited by
-
Immunomodulatory effects of bone marrow-derived mesenchymal stem cells on pro-inflammatory cytokine-stimulated human corneal epithelial cells.PLoS One. 2014 Jul 8;9(7):e101841. doi: 10.1371/journal.pone.0101841. eCollection 2014. PLoS One. 2014. PMID: 25003339 Free PMC article.
-
γδ T Cell-Dependent Regulatory T Cells Prevent the Development of Autoimmune Keratitis.J Immunol. 2015 Dec 15;195(12):5572-81. doi: 10.4049/jimmunol.1501604. Epub 2015 Nov 13. J Immunol. 2015. PMID: 26566677 Free PMC article.
-
CCL20, γδ T cells, and IL-22 in corneal epithelial healing.FASEB J. 2011 Aug;25(8):2659-68. doi: 10.1096/fj.11-184804. Epub 2011 Apr 25. FASEB J. 2011. PMID: 21518851 Free PMC article.
-
A single cell atlas of human cornea that defines its development, limbal progenitor cells and their interactions with the immune cells.Ocul Surf. 2021 Jul;21:279-298. doi: 10.1016/j.jtos.2021.03.010. Epub 2021 Apr 16. Ocul Surf. 2021. PMID: 33865984 Free PMC article.
-
The miR-183/96/182 cluster regulates sensory innervation, resident myeloid cells and functions of the cornea through cell type-specific target genes.Sci Rep. 2024 Apr 1;14(1):7676. doi: 10.1038/s41598-024-58403-1. Sci Rep. 2024. PMID: 38561433 Free PMC article.
References
-
- O’Brien TP, Li Q, Ashraf MF, Matteson DM, Stark WJ, Chan CC. Inflammatory response in the early stages of wound healing after excimer laser keratectomy. Arch Ophthalmol. 1998;116:1470–1474. - PubMed
-
- Wilson SE, Mohan RR, Mohan RR, Ambrosio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–637. - PubMed
-
- Zhu SN, Dana MR. Expression of cell adhesion molecules on limbal and neovascular endothelium in corneal inflammatory neovascularization. Invest Ophthalmol Vis Sci. 1999;40:1427–1434. - PubMed
-
- Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

