Effects of PKA phosphorylation of cardiac troponin I and strong crossbridge on conformational transitions of the N-domain of cardiac troponin C in regulated thin filaments
- PMID: 17676764
- PMCID: PMC2547119
- DOI: 10.1021/bi700574n
Effects of PKA phosphorylation of cardiac troponin I and strong crossbridge on conformational transitions of the N-domain of cardiac troponin C in regulated thin filaments
Abstract
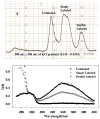
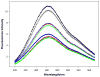
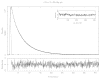
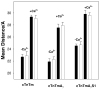
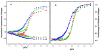
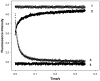
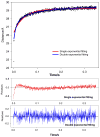
Regulation of cardiac muscle function is initiated by binding of Ca2+ to troponin C (cTnC) which induces a series of structural changes in cTnC and other thin filament proteins. These structural changes are further modulated by crossbridge formation and fine-tuned by phosphorylation of cTnI. The objective of the present study is to use a new Förster resonance energy transfer-based structural marker to distinguish structural and kinetic effects of Ca2+ binding, crossbridge interaction, and protein kinase A phosphorylation of cTnI on the conformational changes of the cTnC N-domain. The FRET-based structural marker was generated by attaching AEDANS to one cysteine of a double-cysteine mutant cTnC(13C/51C) as a FRET donor and attaching DDPM to the other cysteine as the acceptor. The doubly labeled cTnC mutant was reconstituted into the thin filament by adding cTnI, cTnT, tropomyosin, and actin. Changes in the distance between Cys13 and Cys51 induced by Ca2+ binding/dissociation were determined by FRET-sensed Ca2+ titration and stopped-flow studies, and time-resolved fluorescence measurements. The results showed that the presence of both Ca2+ and strong binding of myosin head to actin was required to achieve a fully open structure of the cTnC N-domain in regulated thin filaments. Equilibrium and stopped-flow studies suggested that strongly bound myosin head significantly increased the Ca2+ sensitivity and changed the kinetics of the structural transition of the cTnC N-domain. PKA phosphorylation of cTnI impacted the Ca2+ sensitivity and kinetics of the structural transition of the cTnC N-domain but showed no global structural effect on cTnC opening. These results provide an insight into the modulation mechanism of strong crossbridge and cTnI phosphorylation in cardiac thin filament activation/relaxation processes.
Figures

References
-
- Ebashi S, Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. - PubMed
-
- Farah CS, Reinach FC. The troponin complex and regulation of muscle contraction. FASEB J. 1995;9:755–767. - PubMed
-
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. - PubMed
-
- Dong WJ, Xing J, Villain M, Hellinger M, Robinson JM, Chandra M, Solaro RJ, Umeda PK, Cheung HC. Conformation of the regulatory domain of cardiac muscle troponin C in its complex with cardiac troponin I. J Biol Chem. 1999;274:31382–31390. - PubMed
-
- Li MX, Spyracopoulos L, Sykes BD. Binding of cardiac troponin-I147–163 induces a structural opening in human cardiac troponin-C. Biochemistry. 1999;38:8289–8298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

