Contribution of A1 subunit residue Q316 in thrombin-activated factor VIII to A2 subunit dissociation
- PMID: 17676877
- PMCID: PMC2525606
- DOI: 10.1021/bi700941w
Contribution of A1 subunit residue Q316 in thrombin-activated factor VIII to A2 subunit dissociation
Abstract
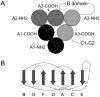
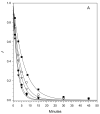
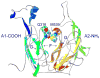
Blood coagulation factor VIII (fVIII) is activated by thrombin to form an A1/A2/A3-C1-C2 heterotrimer, which functions as a cofactor for factor IXa during intrinsic pathway factor X activation. Human thrombin-activated fVIII (fVIIIa) decays rapidly because of first-order dissociation of the A2 subunit, which may function to regulate the coagulation mechanism. The three fVIII A domains each consist of two cupredoxin-like subdomains. Substitution of the COOH-terminal A1 subdomain of porcine fVIIIa, which decays more slowly than human fVIIIa, reduces the dissociation rate constant for fVIIIa decay. Examination of a human fVIII A1-A2-A3 homology model [Pemberton, S., et al. (1997) Blood 89, 2413-2421) revealed a possible interaction between Q316 in the FG helix of the COOH-terminal A1 subdomain and M539 in the FG helix of the NH2-terminal A2 subdomain, which are sites where human and porcine fVIII differ. Decays of purified recombinant human and porcine fVIIIa and the human fVIIIa mutants Q316H, M539L and Q316H/M539L were compared at 23 and 37 degrees C. The decay rates of the Q316H and Q316H/M539L mutants, but not the M539L mutant, were significantly slower than human fVIIIa. These results indicate that the FG helix of the COOH-terminal A1 cupredoxin-like subdomain of fVIII may be under selective pressure by the requirements of hemostatic balance.
Figures


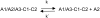
Similar articles
-
A1 subunit-mediated regulation of thrombin-activated factor VIII A2 subunit dissociation.J Biol Chem. 2006 May 19;281(20):13922-30. doi: 10.1074/jbc.M513124200. Epub 2006 Mar 2. J Biol Chem. 2006. PMID: 16513639
-
Hemophilia A mutations associated with 1-stage/2-stage activity discrepancy disrupt protein-protein interactions within the triplicated A domains of thrombin-activated factor VIIIa.Blood. 2001 Feb 1;97(3):685-91. doi: 10.1182/blood.v97.3.685. Blood. 2001. PMID: 11157485
-
Hemophilia A mutations within the factor VIII A2-A3 subunit interface destabilize factor VIIIa and cause one-stage/two-stage activity discrepancy.Thromb Haemost. 2002 Nov;88(5):781-7. Thromb Haemost. 2002. PMID: 12428094
-
The protein structure and effect of factor VIII.Thromb Res. 2007;119(1):1-13. doi: 10.1016/j.thromres.2005.12.015. Epub 2006 Feb 17. Thromb Res. 2007. PMID: 16487577 Review.
-
Role of activation of the coagulation factor VIII in interaction with vWf, phospholipid, and functioning within the factor Xase complex.Trends Cardiovasc Med. 1999 Oct;9(7):185-92. doi: 10.1016/s1050-1738(00)00019-0. Trends Cardiovasc Med. 1999. PMID: 10881749 Review.
Cited by
-
The 3.2 Å structure of a bioengineered variant of blood coagulation factor VIII indicates two conformations of the C2 domain.J Thromb Haemost. 2020 Jan;18(1):57-69. doi: 10.1111/jth.14621. Epub 2019 Sep 8. J Thromb Haemost. 2020. PMID: 31454152 Free PMC article.
-
Gender-Specific Differences in Human Vertebral Bone Marrow Clot.Int J Mol Sci. 2023 Jul 24;24(14):11856. doi: 10.3390/ijms241411856. Int J Mol Sci. 2023. PMID: 37511617 Free PMC article.
-
Stabilizing interactions between D666-S1787 and T657-Y1792 at the A2-A3 interface support factor VIIIa stability in the blood clotting pathway.J Thromb Haemost. 2016 May;14(5):1021-30. doi: 10.1111/jth.13292. Epub 2016 Mar 21. J Thromb Haemost. 2016. PMID: 26878264 Free PMC article.
References
-
- Lollar P, Parker CG. Subunit structure of thrombin-activated porcine factor VIII. Biochemistry. 1989;28:666–674. - PubMed
-
- Lollar P, Parker CG. pH-dependent denaturation of thrombin-activated porcine factor VIII. J Biol Chem. 1990;265:1688–1692. - PubMed
-
- Fay PJ, Haidaris PJ, Smudzin TM. Human factor VIIIa subunit structure: reconstitution of factor VIIIa from the isolated A1/A3-C1-C2 dimer and A2 subunit. J Biol Chem. 1991;266:8957–8962. - PubMed
-
- Hultin MB, Jesty J. The activation and inactivation of human factor VIII by thrombin: effect of inhibitors of thrombin. Blood. 1981;57:476–482. - PubMed
-
- Lollar P, Knutson GJ, Fass DN. Stabilization of thrombin-activated porcine factor VIII:C by factor IXa and phospholipid. Blood. 1984;63:1303–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

