Introduction to computational proteomics
- PMID: 17676979
- PMCID: PMC1933459
- DOI: 10.1371/journal.pcbi.0030114
Introduction to computational proteomics
Conflict of interest statement
Figures





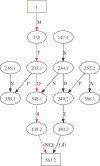
Similar articles
-
Key challenges in proteomics and proteoinformatics. Progress in proteins.IEEE Eng Med Biol Mag. 2005 May-Jun;24(3):34-40. doi: 10.1109/memb.2005.1436456. IEEE Eng Med Biol Mag. 2005. PMID: 15971839 Review. No abstract available.
-
Phosphoproteomics by mass spectrometry and classical protein chemistry approaches.Mass Spectrom Rev. 2005 Nov-Dec;24(6):828-46. doi: 10.1002/mas.20042. Mass Spectrom Rev. 2005. PMID: 15538747 Review.
-
Computational prediction of proteotypic peptides for quantitative proteomics.Nat Biotechnol. 2007 Jan;25(1):125-31. doi: 10.1038/nbt1275. Epub 2006 Dec 31. Nat Biotechnol. 2007. PMID: 17195840
-
Latest developments in sample treatment for 18O-isotopic labeling for proteomics mass spectrometry-based approaches: a critical review.Talanta. 2010 Feb 15;80(4):1476-86. doi: 10.1016/j.talanta.2009.04.053. Epub 2009 May 4. Talanta. 2010. PMID: 20082805
-
Proteomics in 2005/2006: developments, applications and challenges.Anal Chem. 2007 Jun 15;79(12):4325-43. doi: 10.1021/ac070741j. Epub 2007 May 4. Anal Chem. 2007. PMID: 17477510 Review. No abstract available.
Cited by
-
Dispec: a novel peptide scoring algorithm based on peptide matching discriminability.PLoS One. 2013 May 13;8(5):e62724. doi: 10.1371/journal.pone.0062724. Print 2013. PLoS One. 2013. PMID: 23675420 Free PMC article.
-
Simulation Testbed for Evaluating Distributed Querying and Searching of Mass Spectrometry Big Data in a Network-based Infrastructure.Proc (IEEE Int Conf Big Data Comput Serv Appl). 2021 Aug;2021:137-142. doi: 10.1109/bigdataservice52369.2021.00022. Epub 2021 Oct 18. Proc (IEEE Int Conf Big Data Comput Serv Appl). 2021. PMID: 35425943 Free PMC article.
-
Pathway and network analysis in proteomics.J Theor Biol. 2014 Dec 7;362:44-52. doi: 10.1016/j.jtbi.2014.05.031. Epub 2014 Jun 6. J Theor Biol. 2014. PMID: 24911777 Free PMC article. Review.
-
Exploring COVID-19 pathogenesis on command-line: A bioinformatics pipeline for handling and integrating omics data.Adv Protein Chem Struct Biol. 2022;131:311-339. doi: 10.1016/bs.apcsb.2022.04.002. Epub 2022 May 12. Adv Protein Chem Struct Biol. 2022. PMID: 35871895 Free PMC article.
-
A tutorial for software development in quantitative proteomics using PSI standard formats.Biochim Biophys Acta. 2014 Jan;1844(1 Pt A):88-97. doi: 10.1016/j.bbapap.2013.04.004. Epub 2013 Apr 12. Biochim Biophys Acta. 2014. PMID: 23584085 Free PMC article. Review.
References
-
- Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. - PubMed
-
- Pandey A, Mann M. Proteomics to study genes and genomes. Nature. 2000;405:837–846. - PubMed
-
- Patton WF. Proteome analysis. II. Protein subcellular redistribution: Linking physiology to genomics via the proteome and separation technologies involved. J Chromatogr B Analyt Technol Biomed Life Sci. 1999;722:203–223. - PubMed
-
- Khatib-Shahidi S, Andersson M, Herman JL, Gillespie TA, Caprioli RM. Direct molecular analysis of whole-body animal tissue sections by imaging MALDI mass spectrometry. Anal Chem. 2006;78:6448–6456. - PubMed
-
- Gavin AC, Aloy P, Grandi P, Krause R, Boesche M, et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440:631–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

