Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (Melioidosis)
- PMID: 17676990
- PMCID: PMC1950213
- DOI: 10.1371/journal.pmed.0040248
Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (Melioidosis)
Abstract
Background: Toll-like receptors (TLRs) are essential in host defense against pathogens by virtue of their capacity to detect microbes and initiate the immune response. TLR2 is seen as the most important receptor for gram-positive bacteria, while TLR4 is regarded as the gram-negative TLR. Melioidosis is a severe infection caused by the gram-negative bacterium, Burkholderia pseudomallei, that is endemic in Southeast Asia. We aimed to characterize the expression and function of TLRs in septic melioidosis.
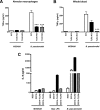
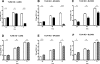
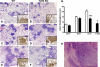
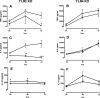
Methods and findings: Patient studies: 34 patients with melioidosis demonstrated increased expression of CD14, TLR1, TLR2, and TLR4 on the cell surfaces of monocytes and granulocytes, and increased CD14, TLR1, TLR2, TLR4, LY96 (also known as MD-2), TLR5, and TLR10 mRNA levels in purified monocytes and granulocytes when compared with healthy controls. In vitro experiments: Whole-blood and alveolar macrophages obtained from TLR2 and TLR4 knockout (KO) mice were less responsive to B. pseudomallei in vitro, whereas in the reverse experiment, transfection of HEK293 cells with either TLR2 or TLR4 rendered these cells responsive to this bacterium. In addition, the lipopolysaccharide (LPS) of B. pseudomallei signals through TLR2 and not through TLR4. Mouse studies: Surprisingly, TLR4 KO mice were indistinguishable from wild-type mice with respect to bacterial outgrowth and survival in experimentally induced melioidosis. In contrast, TLR2 KO mice displayed a markedly improved host defenses as reflected by a strong survival advantage together with decreased bacterial loads, reduced lung inflammation, and less distant-organ injury.
Conclusions: Patients with melioidosis displayed an up-regulation of multiple TLRs in peripheral blood monocytes and granulocytes. Although both TLR2 and TLR4 contribute to cellular responsiveness to B. pseudomallei in vitro, TLR2 detects the LPS of B. pseudomallei, and only TLR2 impacts on the immune response of the intact host in vivo. Inhibition of TLR2 may be a novel treatment strategy in melioidosis.
Conflict of interest statement
Figures

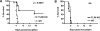
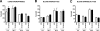

References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. - PubMed
-
- Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: Insights into the pathogenicity of Burkholderia pseudomallei . Nat Rev Microbiol. 2006;4:272–282. - PubMed
-
- Currie BJ. Melioidosis: An important cause of pneumonia in residents of and travellers returned from endemic regions. Eur Respir J. 2003;22:542–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

