The biological basis of audition
- PMID: 17678445
- PMCID: PMC3856181
- DOI: 10.1146/annurev.psych.59.103006.093544
The biological basis of audition
Abstract
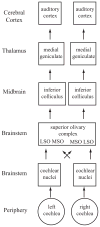
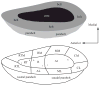
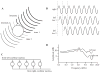
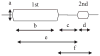
Interest has recently surged in the neural mechanisms of audition, particularly with regard to functional imaging studies in human subjects. This review emphasizes recent work on two aspects of auditory processing. The first explores auditory spatial processing and the role of the auditory cortex in both nonhuman primates and human subjects. The interactions with visual stimuli, the ventriloquism effect, and the ventriloquism aftereffect are also reviewed. The second aspect is temporal processing. Studies investigating temporal integration, forward masking, and gap detection are reviewed, as well as examples from the birdsong system and echolocating bats.
Figures
References
-
- Ahissar M, Ahissar E, Bergman H, Vaadia E. Encoding of sound-source location and movement: activity of single neurons and interactions between adjacent neurons in the monkey auditory cortex. J Neurophysiol. 1992;67:203–15. - PubMed
-
- Alais D, Burr D. The ventriloquist effect results from near-optimal bimodal integration. Curr Biol. 2004;14:257–62. - PubMed
-
- Altmann CF, Bledowski C, Wibral M, Kaiser J. Processing of location and pattern changes of natural sounds in the human auditory cortex. NeuroImage. 2007;35:1192–200. - PubMed
-
- Altshuler MW, Comalli PE. Effect of stimulus intensity and frequency on median horizontal plane sound localization. J Aud Res. 1975;15:262–65.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

