Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease
- PMID: 17678629
- PMCID: PMC2254136
- DOI: 10.1016/j.bcp.2007.07.004
Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease
Abstract
Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that degrade various components of the extracellular matrix (ECM). Members of the MMP family include collagenases, gelatinases, stromelysins, matrilysins and membrane-type MMPs. ProMMPs are cleaved into active forms that promote degradation of ECM proteins. Also, recent evidence suggests direct or indirect effects of MMPs on ion channels in the endothelium and vascular smooth muscle, and on other mechanisms of vascular relaxation/contraction. Endogenous tissue inhibitors of metalloproteinases (TIMPs) reduce excessive proteolytic ECM degradation by MMPs. The balance between MMPs and TIMPs plays a major role in vascular remodeling, angiogenesis, and the uterine and systemic vasodilation during normal pregnancy. An imbalance in the MMPs/TIMPs activity ratio may underlie the pathogenesis of vascular diseases such as abdominal aortic aneurysm, varicose veins, hypertension and preeclampsia. Downregulation of MMPs using genetic manipulations of endogenous TIMPs, or synthetic pharmacological inhibitors such as BB-94 (Batimastat) and doxycycline, and Ro-28-2653, a more specific inhibitor of gelatinases and membrane type 1-MMP, could be beneficial in reducing the MMP-mediated vascular dysfunction and the progressive vessel wall damage associated with vascular disease.
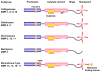
Figures
References
-
- Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension. 1993;21:391–397. - PubMed
-
- Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension. 1996;28:505–506. - PubMed
-
- Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci. 2002;17:105–109. - PubMed
-
- De Mey JG, Schiffers PM, Hilgers RH, Sanders MM. Toward functional genomics of flow-induced outward remodeling of resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288(3):H1022–H1027. - PubMed
-
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

