Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity
- PMID: 17678736
- PMCID: PMC2239316
- DOI: 10.1016/j.jacc.2007.03.057
Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity
Abstract
Objectives: We aimed to explore the effects of pharmacologic inhibition of cannabinoid-1 (CB1) receptor in in vivo and in vitro models of doxorubicin (DOX)-induced cardiotoxicity.
Background: Doxorubicin is one of the most potent antitumor agents available; however, its clinical use is limited because of the risk of severe cardiotoxicity. Endocannabinoids mediate cardiodepressive effects through CB1 receptors in various pathophysiological conditions, and these effects can be reversed by CB1 antagonists.
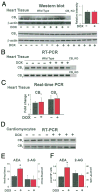
Methods: Left ventricular function was measured by Millar pressure-volume system. Apoptosis markers, CB1/CB2 receptor expression, and endocannabinoid levels were determined by immunohistochemistry, Western blot, reverse transcription-polymerase chain reaction, real-time polymerase chain reaction, flow cytometry, fluorescent microscopy, and liquid chromatography/in-line mass spectrometry techniques.
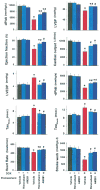
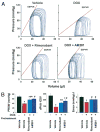
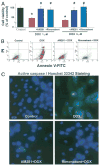
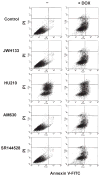
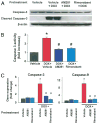
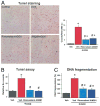
Results: Five days after the administration of a single dose of DOX (20 mg/kg intraperitoneally) to mice, left ventricular systolic pressure, maximum first derivative of ventricular pressure with respect to time (+dP/dt), stroke work, ejection fraction, cardiac output, and load-independent indexes of contractility (end-systolic pressure-volume relation, preload-recruitable stroke work, dP/dt-end-diastolic volume relation) were significantly depressed, and the myocardial level of the endocannabinoid anandamide (but not CB1/CB2 receptor expression) was elevated compared with vehicle-treated control mice. Treatment with the CB1 antagonists rimonabant or AM281 markedly improved cardiac dysfunction and reduced DOX-induced apoptosis in the myocardium. Doxorubicin also decreased cell viability and induced apoptosis in the H9c2 myocardial cell line measured by flow cytometry and fluorescent microscopy, which were prevented by the preincubation of the cells with either CB1 antagonist, but not with CB1 and CB2 agonists and CB2 antagonists.
Conclusions: These data suggest that CB1 antagonists may represent a new cardioprotective strategy against DOX-induced cardiotoxicity.
Figures
Comment in
-
Endocannabinoid inhibition: a new cardioprotective strategy against doxorubicin cardiotoxicity.J Am Coll Cardiol. 2007 Aug 7;50(6):537-9. doi: 10.1016/j.jacc.2007.04.052. Epub 2007 Jul 23. J Am Coll Cardiol. 2007. PMID: 17678737 No abstract available.
References
-
- Young RC, Ozols RF, Myers CE. The anthracycline antineoplastic drugs. N Engl J Med. 1981;305:139–53. - PubMed
-
- Hortobagyi GN. Anthracyclines in the treatment of cancer. An overview Drugs. 1997;54(Suppl 4):1–7. - PubMed
-
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–5. - PubMed
-
- Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977;197:165–7. - PubMed
-
- Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

