Anterior regions of monkey parietal cortex process visual 3D shape
- PMID: 17678860
- PMCID: PMC3011365
- DOI: 10.1016/j.neuron.2007.06.040
Anterior regions of monkey parietal cortex process visual 3D shape
Abstract
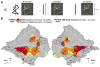
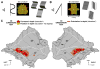
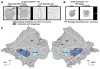
The intraparietal cortex is involved in the control of visually guided actions, like reach-to-grasp movements, which require extracting the 3D shape and position of objects from 2D retinal images. Using fMRI in behaving monkeys, we investigated the role of the intraparietal cortex in processing stereoscopic information for recovering the depth structure and the position in depth of objects. We found that while several areas (CIP, LIP, and AIP on the lateral bank; PIP and MIP on the medial bank) are activated by stereoscopic stimuli, AIP and an adjoining portion of LIP are sensitive only to depth structure. Furthermore, only these two regions are sensitive to both the depth structure and the 2D shape of small objects. These results indicate that extracting 3D spatial information from stereo involves several intraparietal areas, among which AIP and anterior LIP are more specifically engaged in extracting the 3D shape of objects.
Figures




References
-
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. - PubMed
-
- Baker JT, Patel GH, Corbetta M, Snyder LH. Distribution of activity across the monkey cerebral cortical surface, thalamus and midbrain during rapid, visually guided saccades. Cereb Cortex. 2006;16:447–459. - PubMed
-
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: A quantitative receptive field analysis. Exp Brain Res. 2001;140:127–144. - PubMed
-
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intra-parietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. - PubMed
-
- Boltz RL, Harwerth RS. Fusional vergence ranges of the monkey: A behavioral study. Exp Brain Res. 1979;37:87–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

