The bactericidal/permeability-increasing protein (BPI) in infection and inflammatory disease
- PMID: 17678885
- PMCID: PMC2695927
- DOI: 10.1016/j.cca.2007.07.005
The bactericidal/permeability-increasing protein (BPI) in infection and inflammatory disease
Abstract
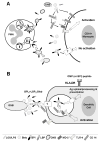
Gram-negative bacteria (GNB) and their endotoxin present a constant environmental challenge. Endotoxins can potently signal mobilization of host defenses against invading GNB but also potentially induce severe pathophysiology, necessitating controlled initiation and resolution of endotoxin-induced inflammation to maintain host integrity. The bactericidal/permeability-increasing protein (BPI) is a pluripotent protein expressed, in humans, mainly neutrophils. BPI exhibits strong antimicrobial activity against GNB and potent endotoxin-neutralizing activity. BPI mobilized with neutrophils in response to invading GNB can promote intracellular and extracellular bacterial killing, endotoxin neutralization and clearance, and delivery of GNB outer membrane antigens to dendritic cells. Tissue expression by dermal fibroblasts and epithelia could further amplify local levels of BPI and local interaction with GNB and endotoxin, helping to constrain local tissue infection and inflammation and prevent systemic infection and systemic inflammation. This review article focuses on the structural and functional properties of BPI with respect to its contribution to host defense during GNB infections and endotoxin-induced inflammation and the genesis of autoantibodies against BPI that can blunt BPI activity and potentially contribute to chronic inflammatory disease.
Conflict of interest statement
Figures
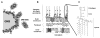



Similar articles
-
From infection to autoimmunity: a new model for induction of ANCA against the bactericidal/permeability increasing protein (BPI).Autoimmun Rev. 2007 Mar;6(4):223-7. doi: 10.1016/j.autrev.2006.08.005. Epub 2006 Sep 5. Autoimmun Rev. 2007. PMID: 17317612 Review.
-
A novel role for the bactericidal/permeability increasing protein in interactions of gram-negative bacterial outer membrane blebs with dendritic cells.J Immunol. 2007 Aug 15;179(4):2477-84. doi: 10.4049/jimmunol.179.4.2477. J Immunol. 2007. PMID: 17675509
-
The endotoxin-binding bactericidal/permeability-increasing protein (BPI): a target antigen of autoantibodies.J Leukoc Biol. 2001 Apr;69(4):505-12. J Leukoc Biol. 2001. PMID: 11310835 Review.
-
Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defence against Gram-negative bacteria.Biochem Soc Trans. 2003 Aug;31(Pt 4):785-90. doi: 10.1042/bst0310785. Biochem Soc Trans. 2003. PMID: 12887306 Review.
-
Relative concentrations of endotoxin-binding proteins in body fluids during infection.Lancet. 1994 Aug 13;344(8920):429-31. doi: 10.1016/s0140-6736(94)91767-1. Lancet. 1994. PMID: 7520106
Cited by
-
A theoretical approach to spot active regions in antimicrobial proteins.BMC Bioinformatics. 2009 Nov 11;10:373. doi: 10.1186/1471-2105-10-373. BMC Bioinformatics. 2009. PMID: 19906288 Free PMC article.
-
Novel heterozygous BPIFC variant in a Chinese pedigree with hereditary trichilemmal cysts.Mol Genet Genomic Med. 2019 Jun;7(6):e697. doi: 10.1002/mgg3.697. Epub 2019 Apr 29. Mol Genet Genomic Med. 2019. PMID: 31033252 Free PMC article.
-
Gene Expression in Embryos From Norwegian Red Bulls With High or Low Non Return Rate: An RNA-Seq Study of in vivo-Produced Single Embryos.Front Genet. 2022 Jan 14;12:780113. doi: 10.3389/fgene.2021.780113. eCollection 2021. Front Genet. 2022. PMID: 35096004 Free PMC article.
-
Identification of probable early-onset biomarkers for tuberculosis disease progression.PLoS One. 2011;6(9):e25230. doi: 10.1371/journal.pone.0025230. Epub 2011 Sep 23. PLoS One. 2011. PMID: 21966464 Free PMC article.
-
B cell responses in older adults with latent tuberculosis: Considerations for vaccine development.Glob Vaccines Immunol. 2016 Jun;1(2):44-52. doi: 10.15761/GVI.1000112. Epub 2016 May 27. Glob Vaccines Immunol. 2016. PMID: 30271881 Free PMC article.
References
-
- Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–202. - PubMed
-
- Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–76. - PubMed
-
- Munford RS. Detoxifying endotoxin: time, place and person. J Endotoxin Res. 2005;11:69–84. - PubMed
-
- Dellinger RP. Inflammation and coagulation: implications for the septic patient. Clin Infect Dis. 2003;36:1259–65. - PubMed
-
- Bainton DF. Neutrophilic leukocyte granules: from structure to function. Adv Exp Med Biol. 1993;336:17–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

