APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase
- PMID: 17679094
- PMCID: PMC2000825
- DOI: 10.1016/j.molcel.2007.06.013
APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase
Abstract
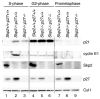
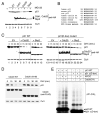
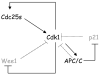
During the G1/S transition, p21 proteolysis is mediated by Skp2; however, p21 reaccumulates in G2 and is degraded again in prometaphase. How p21 degradation is controlled in mitosis remains unexplored. We found that Cdc20 (an activator of the ubiquitin ligase APC/C) binds p21 in cultured cells and identified a D box motif in p21 necessary for APC/C(Cdc20)-mediated ubiquitylation of p21. Overexpression of Cdc20 or Skp2 destabilized wild-type p21; however, only Skp2, but not Cdc20, was able to destabilize a p21(D box) mutant. Silencing of Cdc20 induced an accumulation of p21, increased the fraction of p21 bound to Cdk1, and inhibited Cdk1 activity in p21(+/+) prometaphase cells, but not in p21(-/-) cells. Thus, in prometaphase Cdc20 positively regulates Cdk1 by mediating the degradation of p21. We propose that the APC/C(Cdc20)-mediated degradation of p21 contributes to the full activation of Cdk1 necessary for mitotic events and prevents mitotic slippage during spindle checkpoint activation.
Figures




References
-
- Acquaviva C, Pines J. The anaphase-promoting complex/cyclosome: APC/C. J Cell Sci. 2006;119:2401–2404. - PubMed
-
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. - PubMed
-
- Bates S, Ryan KM, Phillips AC, Vousden KH. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene. 1998;17:1691–1703. - PubMed
-
- Bloom J, Amador V, Bartolini F, DeMartino G, Pagano M. Proteasome-Mediated Degradation of p21 via N-Terminal Ubiquitinylation. Cell. 2003;115:71–82. - PubMed
-
- Bloom J, Pagano M. To be or not to be ubiquitinated? Cell Cycle. 2004;3:138–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

