Halothane increases non-vesicular [(3)H]dopamine release from brain cortical slices
- PMID: 17680357
- PMCID: PMC11517219
- DOI: 10.1007/s10571-007-9162-0
Halothane increases non-vesicular [(3)H]dopamine release from brain cortical slices
Abstract
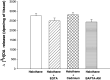
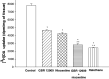
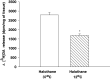
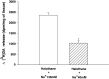
Experimental data suggest that halothane anesthesia is associated with significant changes in dopamine (DA) concentration in some brain regions but the mechanism of this effect is not well known. Rat brain cortical slices were labeled with [(3)H]DA to further characterize the effects of halothane on the release of this neurotransmitter from the central nervous system. Halothane induced an increase on the release of [(3)H]DA that was dependent on incubation time and anesthetic concentration (0.012, 0.024, 0.048, 0.072 and 0.096 mM). This effect was independent of extracellular or intracellular calcium. In addition, [(3)H]DA release evoked by halothane was not affected by TTX (blocker of voltage-dependent Na(+) channels) or reserpine (a blocker of vesicular monoamine transporter). These data suggest that [(3)H]DA release induced by halothane is non-vesicular and would be mediated by the dopamine transporter (DAT) and norepinephrine transporter (NET). GBR 12909 and nomifensine, inhibitors of DAT, decreased the release of [(3)H]DA evoked by halothane. Nisoxetine, a blocker of NET, reduced the release of [(3)H]DA induced by halothane. In addition, GBR 12909, nisoxetine and, halothane decrease the uptake of [(3)H]DA into rat brain cortical slices. A decrease on halothane-induced release of [(3)H]DA was also observed when the brain cortical slices were incubated at low temperature and low extracellular sodium, which are known to interfere with the carrier-mediated release of the neurotransmitter. Ouabain, a Na(+)/K(+) ATPase pump inhibitor, which induces DA release through reverse transport, decreased [(3)H]DA release induced by halothane. It is suggested that halothane increases [(3)H]DA release in brain cortical slices that is mediated by DAT and NET present in the plasma membrane.
Figures





References
-
- Adachi YU, Watanabe K, Satoh T, Vizi ES (2001a) Halothane potentiates the effect of methamphetamine and nomifensine on extracellular dopamine level in rat striatum. Br J Anaesth 86:837–845 - PubMed
-
- Adachi YU, Watanabe K, Higuchi H, Satoh T, Zsilla G (2001b) Halothane decreases impulse-dependent but not cytoplasmic release of dopamine from rat striatal slices. Brain Res Bull 56:521–524 - PubMed
-
- Adachi YU, Yamada S, Satomoto M, Higuchi H, Watanabe K, Kazama T (2005) Isoflurane anesthesia induces biphasic effect on dopamine release in the rat striatum. Brain Res Bull 67:176–181 - PubMed
-
- Andersen PH (1989) The dopamine inhibitor GBR 12909. Selectivity and molecular mechanism of action. Eur J Pharmacol 166:493–504 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

