Differential modulation of Alzheimer's disease amyloid beta-peptide accumulation by diverse classes of metal ligands
- PMID: 17680773
- PMCID: PMC2275059
- DOI: 10.1042/BJ20070579
Differential modulation of Alzheimer's disease amyloid beta-peptide accumulation by diverse classes of metal ligands
Abstract
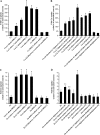
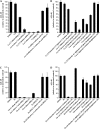
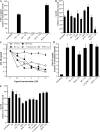
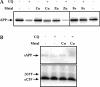
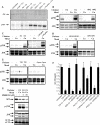
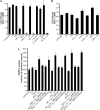
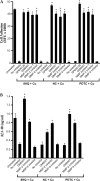
Biometals have an important role in AD (Alzheimer's disease) and metal ligands have been investigated as potential therapeutic agents for treatment of AD. In recent studies the 8HQ (8-hydroxyquinoline) derivative CQ (clioquinol) has shown promising results in animal models and small clinical trials; however, the actual mode of action in vivo is still being investigated. We previously reported that CQ-metal complexes up-regulated MMP (matrix metalloprotease) activity in vitro by activating PI3K (phosphoinositide 3-kinase) and JNK (c-jun N-terminal kinase), and that the increased MMP activity resulted in enhanced degradation of secreted Abeta (amyloid beta) peptide. In the present study, we have further investigated the biochemical mechanisms by which metal ligands affect Abeta metabolism. To achieve this, we measured the effects of diverse metal ligands on cellular metal uptake and secreted Abeta levels in cell culture. We report that different classes of metal ligands including 8HQ and phenanthroline derivatives and the sulfur compound PDTC (pyrrolidine dithiocarbamate) elevated cellular metal levels (copper and zinc), and resulted in substantial loss of secreted Abeta. Generally, the ability to inhibit Abeta levels correlated with a higher lipid solubility of the ligands and their capacity to increase metal uptake. However, we also identified several ligands that potently inhibited Abeta levels while only inducing minimal change to cellular metal levels. Metal ligands that inhibited Abeta levels [e.g. CQ, 8HQ, NC (neocuproine), 1,10-phenanthroline and PDTC] induced metal-dependent activation of PI3K and JNK, resulting in JNK-mediated up-regulation of metalloprotease activity and subsequent loss of secreted Abeta. The findings in the present study show that diverse metal ligands with high lipid solubility can elevate cellular metal levels resulting in metalloprotease-dependent inhibition of Abeta. Given that a structurally diverse array of ligands was assessed, the results are consistent with the effects being due to metal transport rather than the chelating ligand interacting directly with a receptor.
Figures

References
-
- Bush A. I., Pettingell W. H., Multhaup G., d Paradis M., Vonsattel J. P., Gusella J. F., Beyreuther K., Masters C. L., Tanzi R. E. Rapid induction of Alzheimer A β amyloid formation by zinc. Science. 1994;265:1464–1467. - PubMed
-
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120:885–890. - PubMed
-
- Atwood C. S., Scarpa R. C., Huang X., Moir R. D., Jones W. D., Fairlie D. P., Tanzi R. E., Bush A. I. Characterization of copper interactions with Alzheimer amyloid β peptides: identification of an attomolar-affinity copper binding site on amyloid β1–42. J. Neurochem. 2000;75:1219–1233. - PubMed
-
- Bush A. I., Multhaup G., Moir R. D., Williamson T. G., Small D. H., Rumble B., Pollwein P., Beyreuther K., Masters C. L. A novel zinc(II) binding site modulates the function of the βA4 amyloid protein precursor of Alzheimer's disease. J. Biol. Chem. 1993;268:16109–16112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

