Role of osteopontin in neutrophil function
- PMID: 17680800
- PMCID: PMC2266047
- DOI: 10.1111/j.1365-2567.2007.02682.x
Role of osteopontin in neutrophil function
Abstract
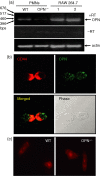
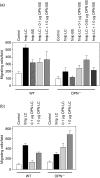
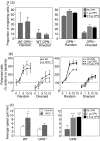
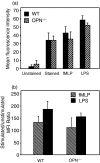
Osteopontin (OPN) is important for the function of fibroblasts, macrophages and lymphocytes during inflammation and wound healing. In recent studies of experimental colitis we demonstrated exacerbated tissue destruction in OPN-null mice, associated with reduced tumour necrosis factor-alpha expression and increased myeloperoxidase activity. The objective of this investigation therefore was to determine the importance of OPN expression in neutrophil function. Although, in contrast to macrophages, neutrophils expressed low levels of OPN with little or no association with the CD44 receptor, intraperitoneal recruitment of neutrophils in OPN-null mice was impaired in response to sodium periodate. The importance of exogenous OPN for neutrophil recruitment was demonstrated by a robust increase in peritoneal infiltration of PMNs in response to injections of native or recombinant OPN. In vitro, OPN(-/-) neutrophils exhibited reduced chemokinesis and chemotaxis towards N-formyl methionyl leucyl phenylalanine (fMLP), reflecting a reduction in migration speed and polarization. Exogenous OPN, which was chemotactic for the neutrophils, rescued the defects in polarization and migration speed of the OPN(-/-) neutrophils. In contrast, the defensive and cytocidal activities of OPN(-/-) neutrophils, measured by assays for phagocytosis, generation of reactive oxygen species, cytokine production and matrix metalloproteinase-9, were not impaired. These studies demonstrate that, while exogenous OPN may be important for the recruitment and migration of neutrophils, expression of OPN by neutrophils is not required for their destructive capabilities.
Figures



Similar articles
-
Osteopontin undergoes polymerization in vivo and gains chemotactic activity for neutrophils mediated by integrin alpha9beta1.J Biol Chem. 2011 Apr 1;286(13):11170-8. doi: 10.1074/jbc.M110.189258. Epub 2011 Feb 14. J Biol Chem. 2011. PMID: 21321126 Free PMC article.
-
Osteopontin is up-regulated and associated with neutrophil and macrophage infiltration in glioblastoma.Immunology. 2011 Jan;132(1):39-48. doi: 10.1111/j.1365-2567.2010.03335.x. Epub 2010 Aug 17. Immunology. 2011. PMID: 20722758 Free PMC article.
-
Osteopontin as two-sided mediator of intestinal inflammation.J Cell Mol Med. 2009 Jun;13(6):1162-74. doi: 10.1111/j.1582-4934.2008.00428.x. Epub 2008 Jul 4. J Cell Mol Med. 2009. PMID: 18627421 Free PMC article.
-
Modulation of infection-mediated migration of neutrophils and CXCR2 trafficking by osteopontin.Immunology. 2017 Jan;150(1):74-86. doi: 10.1111/imm.12668. Epub 2016 Oct 7. Immunology. 2017. PMID: 27599164 Free PMC article.
-
Role of osteopontin in systemic lupus erythematosus.Arch Immunol Ther Exp (Warsz). 2014 Dec;62(6):475-82. doi: 10.1007/s00005-014-0294-x. Epub 2014 Jun 11. Arch Immunol Ther Exp (Warsz). 2014. PMID: 24917428 Free PMC article. Review.
Cited by
-
Osteopontin undergoes polymerization in vivo and gains chemotactic activity for neutrophils mediated by integrin alpha9beta1.J Biol Chem. 2011 Apr 1;286(13):11170-8. doi: 10.1074/jbc.M110.189258. Epub 2011 Feb 14. J Biol Chem. 2011. PMID: 21321126 Free PMC article.
-
Osteopontin facilitates ultraviolet B-induced squamous cell carcinoma development.J Dermatol Sci. 2014 Aug;75(2):121-32. doi: 10.1016/j.jdermsci.2014.05.002. Epub 2014 May 21. J Dermatol Sci. 2014. PMID: 24888687 Free PMC article.
-
Cumulative effects of bone and soft tissue injury on systemic inflammation: a pilot study.Clin Orthop Relat Res. 2013 Sep;471(9):2815-21. doi: 10.1007/s11999-013-2908-8. Clin Orthop Relat Res. 2013. PMID: 23479238 Free PMC article.
-
Osteopontin is up-regulated and associated with neutrophil and macrophage infiltration in glioblastoma.Immunology. 2011 Jan;132(1):39-48. doi: 10.1111/j.1365-2567.2010.03335.x. Epub 2010 Aug 17. Immunology. 2011. PMID: 20722758 Free PMC article.
-
Effects of enamel matrix derivative on non-surgical management of peri-implant mucositis: a double-blind randomized clinical trial.Clin Oral Investig. 2017 Sep;21(7):2379-2388. doi: 10.1007/s00784-016-2033-7. Epub 2016 Dec 30. Clin Oral Investig. 2017. PMID: 28039545 Clinical Trial.
References
-
- Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–22. - PubMed
-
- Sodek J, Batista Da Silva AP, Zohar R. Osteopontin and mucosal protection. J Dent Res. 2006;85:404–15. - PubMed
-
- da Silva AP, Pollett A, Rittling SR, Denhardt DT, Sodek J, Zohar R. Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-alpha expression and non-programmed cell death. J Cell Physiol. 2006;208:629–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

