Genetic findings in Parkinson's disease and translation into treatment: a leading role for mitochondria?
- PMID: 17680806
- PMCID: PMC2268956
- DOI: 10.1111/j.1601-183X.2007.00342.x
Genetic findings in Parkinson's disease and translation into treatment: a leading role for mitochondria?
Abstract
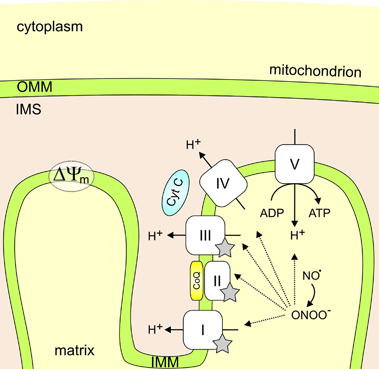
Parkinson's disease (PD) is a progressive neurodegenerative movement disorder and in most patients its aetiology remains unknown. Molecular genetic studies in familial forms of the disease identified key proteins involved in PD pathogenesis, and support a major role for mitochondrial dysfunction, which is also of significant importance to the common sporadic forms of PD. While current treatments temporarily alleviate symptoms, they do not halt disease progression. Drugs that target the underlying pathways to PD pathogenesis, including mitochondrial dysfunction, therefore hold great promise for neuroprotection in PD. Here we summarize how the proteins identified through genetic research (alpha-synuclein, parkin, PINK1, DJ-1, LRRK2 and HTRA2) fit into and add to our current understanding of the role of mitochondrial dysfunction in PD. We highlight how these genetic findings provided us with suitable animal models and critically review how the gained insights will contribute to better therapies for PD.
Figures

References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JMG, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, Wood NW. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann Neurol. 2003;54:283–286. - PubMed
-
- Abou-Sleiman PM, Healy DG, Wood NW. Causes of Parkinson’s disease: genetics of DJ-1. Cell Tissue Res. 2004;318:185–188. - PubMed
-
- Abou-Sleiman PM, Muqit MMK, McDonald NQ, Yang YX, Gandhi S, Healy DG, Harvey K, Harvey RJ, Deas E, Hatia K, Quinn N, Lees A, Latchman DS, Wood NW. A heterozygous effect for PINK1 mutations in Parkinson’s disease? Ann Neurol. 2006;60:414–419. - PubMed
-
- Akao Y, Maruyama W, Yi H, Shamoto-Nagai M, Youdim MBH, Naoi M. An anti-Parkinson’s disease drug, N-propargyl-1(R)-aminoindan (rasagiline), enhances expression of anti-apoptotic Bcl-2 in human dopaminergic SH-SY5Y cells. Neurosci Lett. 2002;326:105–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

