Hepatic proprotein convertases modulate HDL metabolism
- PMID: 17681148
- PMCID: PMC2565575
- DOI: 10.1016/j.cmet.2007.07.009
Hepatic proprotein convertases modulate HDL metabolism
Abstract
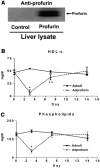
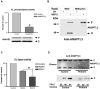
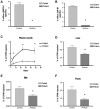
The risk of atherosclerosis is inversely associated with plasma levels of high-density lipoprotein cholesterol (HDL-C). However, HDL metabolism is incompletely understood, and there are few effective approaches to modulate HDL-C levels. Here we show that inhibition in the liver of the classical proprotein convertases (PCs), but not the atypical PCs S1P and PCSK9, decreases plasma HDL-C levels. This metabolic effect of hepatic PCs is critically dependent on expression of endothelial lipase (EL), an enzyme that directly hydrolyzes HDL phospholipids and promotes its catabolism. Hepatic PCs reduce EL function through direct inactivating cleavage of EL as well as through activating cleavage of angiopoietin-like protein 3 (ANGPTL3), an endogenous inhibitor of EL. Thus, inhibition of hepatic PCs results in increased EL activity, leading to reduced HDL-C as well as impaired reverse cholesterol transport. The hepatic PC-ANGPTL3-EL-HDL pathway is therefore a novel mechanism controlling HDL metabolism and cholesterol homeostasis.
Figures

References
-
- Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003;34:154–156. - PubMed
-
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. - PubMed
-
- Basak A. Inhibitors of proprotein convertases. J. Mol. Med. 2005;83:844–855. - PubMed
-
- Benjannet S, Elagoz A, Wickham L, Mamarbachi M, Munzer JS, Basak A, Lazure C, Cromlish JA, Sisodia S, Checler F, et al. Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J. Biol. Chem. 2001;276:10879–10887. - PubMed
-
- Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, Asselin MC, Hamelin J, Varret M, Allard D, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 2004;279:48865–48875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

