MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer
- PMID: 17681183
- PMCID: PMC4285346
- DOI: 10.1053/j.gastro.2007.05.022
MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer
Abstract
Background and aims: microRNAs (miRNAs) are short noncoding RNAs that regulate gene expression negatively. Although a role for aberrant miRNA expression in cancer has been postulated, the pathophysiologic role and relevance of aberrantly expressed miRNA to tumor biology has not been established.
Methods: We evaluated the expression of miRNA in human hepatocellular cancer (HCC) by expression profiling, and defined a target gene and biologically functional effect of an up-regulated miRNA.
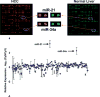
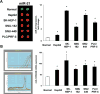
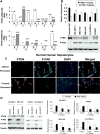
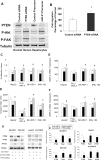
Results: miR-21 was noted to be highly overexpressed in HCC tumors and cell lines in expression profiling studies using a miRNA microarray. Inhibition of miR-21 in cultured HCC cells increased expression of the phosphatase and tensin homolog (PTEN) tumor suppressor, and decreased tumor cell proliferation, migration, and invasion. In contrast-enhanced miR-21 expression by transfection with precursor miR-21 increased tumor cell proliferation, migration, and invasion. Moreover, an increase in cell migration was observed in normal human hepatocytes transfected with precursor miR-21. PTEN was shown to be a direct target of miR-21, and to contribute to miR-21 effects on cell invasion. Modulation of miR-21 altered focal adhesion kinase phosphorylation and expression of matrix metalloproteases 2 and 9, both downstream mediators of PTEN involved in cell migration and invasion.
Conclusions: Aberrant expression of miR-21 can contribute to HCC growth and spread by modulating PTEN expression and PTEN-dependent pathways involved in mediating phenotypic characteristics of cancer cells such as cell growth, migration, and invasion.
Figures

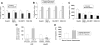
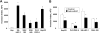

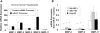
References
-
- El Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. - PubMed
-
- Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Thorgeirsson SS, Lee JS, Grisham JW. Functional genomics of hepatocellular carcinoma. Hepatology. 2006;43:S145–S150. - PubMed
-
- Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390–7394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

