Mannheimia haemolytica leukotoxin binds to lipid rafts in bovine lymphoblastoid cells and is internalized in a dynamin-2- and clathrin-dependent manner
- PMID: 17682044
- PMCID: PMC2044511
- DOI: 10.1128/IAI.00534-07
Mannheimia haemolytica leukotoxin binds to lipid rafts in bovine lymphoblastoid cells and is internalized in a dynamin-2- and clathrin-dependent manner
Abstract
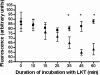
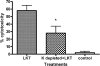
Mannheimia haemolytica is the principal bacterial pathogen of the bovine respiratory disease complex. Its most important virulence factor is a leukotoxin (LKT), which is a member of the RTX family of exotoxins produced by many gram-negative bacteria. Previous studies demonstrated that LKT binds to the beta(2)-integrin LFA-1 (CD11a/CD18) on bovine leukocytes, resulting in cell death. In this study, we demonstrated that depletion of lipid rafts significantly decreases LKT-induced bovine lymphoblastoid cell (BL-3) death. After binding to BL-3 cells, some of the LKT relocated to lipid rafts in an LFA-1-independent manner. We hypothesized that after binding to LFA-1 on BL-3 cells, LKT moves to lipid rafts and clathrin-coated pits via a dynamic process that results in LKT internalization and cytotoxicity. Knocking down dynamin-2 by small interfering RNA reduced both LKT internalization and cytotoxicity. Similarly, expression of dominant negative Eps15 protein expression, which is required for clathrin coat formation, reduced LKT internalization and LKT-mediated cytotoxicity to BL-3 cells. Finally, we demonstrated that inhibiting actin polymerization reduced both LKT internalization and LKT-mediated cytotoxicity. These results suggest that both lipid rafts and clathrin-mediated mechanisms are important for LKT internalization and cytotoxicity in BL-3 cells and illustrate the complex nature of LKT internalization by the cytoskeletal network.
Figures






References
-
- Ambagala, T. C., A. P. Ambagala, and S. Srikumaran. 1999. The leukotoxin of Pasteurella haemolytica binds to beta(2) integrins on bovine leukocytes. FEMS Microbiol. Lett. 179:161-167. - PubMed
-
- Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

