Conformational changes in the GTPase modules of the signal reception particle and its receptor drive initiation of protein translocation
- PMID: 17682051
- PMCID: PMC2064468
- DOI: 10.1083/jcb.200702018
Conformational changes in the GTPase modules of the signal reception particle and its receptor drive initiation of protein translocation
Abstract
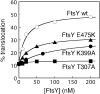
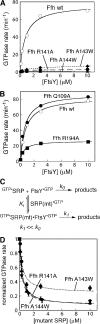
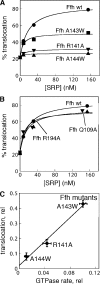
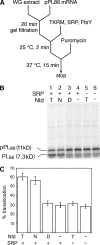
During cotranslational protein targeting, two guanosine triphosphatase (GTPase) in the signal recognition particle (SRP) and its receptor (SR) form a unique complex in which hydrolyses of both guanosine triphosphates (GTP) are activated in a shared active site. It was thought that GTP hydrolysis drives the recycling of SRP and SR, but is not crucial for protein targeting. Here, we examined the translocation efficiency of mutant GTPases that block the interaction between SRP and SR at specific stages. Surprisingly, mutants that allow SRP-SR complex assembly but block GTPase activation severely compromise protein translocation. These mutations map to the highly conserved insertion box domain loops that rearrange upon complex formation to form multiple catalytic interactions with the two GTPs. Thus, although GTP hydrolysis is not required, the molecular rearrangements that lead to GTPase activation are essential for protein targeting. Most importantly, our results show that an elaborate rearrangement within the SRP-SR GTPase complex is required to drive the unloading and initiate translocation of cargo proteins.
Figures


Similar articles
-
Multi-state targeting machinery govern the fidelity and efficiency of protein localization.Adv Exp Med Biol. 2014;805:385-409. doi: 10.1007/978-3-319-02970-2_16. Adv Exp Med Biol. 2014. PMID: 24446370
-
Multiple conformational switches in a GTPase complex control co-translational protein targeting.Proc Natl Acad Sci U S A. 2009 Feb 10;106(6):1754-9. doi: 10.1073/pnas.0808573106. Epub 2009 Jan 27. Proc Natl Acad Sci U S A. 2009. PMID: 19174514 Free PMC article.
-
Co-translational protein targeting by the signal recognition particle.FEBS Lett. 2005 Feb 7;579(4):921-6. doi: 10.1016/j.febslet.2004.11.049. FEBS Lett. 2005. PMID: 15680975 Review.
-
Substrate twinning activates the signal recognition particle and its receptor.Nature. 2004 Jan 15;427(6971):215-21. doi: 10.1038/nature02250. Nature. 2004. PMID: 14724630
-
A tale of two GTPases in cotranslational protein targeting.Protein Sci. 2011 Nov;20(11):1790-5. doi: 10.1002/pro.729. Epub 2011 Sep 27. Protein Sci. 2011. PMID: 21898651 Free PMC article. Review.
Cited by
-
Transient tether between the SRP RNA and SRP receptor ensures efficient cargo delivery during cotranslational protein targeting.Proc Natl Acad Sci U S A. 2010 Apr 27;107(17):7698-703. doi: 10.1073/pnas.1002968107. Epub 2010 Apr 12. Proc Natl Acad Sci U S A. 2010. PMID: 20385832 Free PMC article.
-
Two-step membrane binding by the bacterial SRP receptor enable efficient and accurate Co-translational protein targeting.Elife. 2017 Jul 28;6:e25885. doi: 10.7554/eLife.25885. Elife. 2017. PMID: 28753124 Free PMC article.
-
Evolution from the prokaryotic to the higher plant chloroplast signal recognition particle: the signal recognition particle RNA is conserved in plastids of a wide range of photosynthetic organisms.Plant Cell. 2012 Dec;24(12):4819-36. doi: 10.1105/tpc.112.102996. Epub 2012 Dec 28. Plant Cell. 2012. PMID: 23275580 Free PMC article.
-
Sequential checkpoints govern substrate selection during cotranslational protein targeting.Science. 2010 May 7;328(5979):757-60. doi: 10.1126/science.1186743. Science. 2010. PMID: 20448185 Free PMC article.
-
YlxM is a newly identified accessory protein that influences the function of signal recognition particle pathway components in Streptococcus mutans.J Bacteriol. 2014 Jun;196(11):2043-52. doi: 10.1128/JB.01465-13. Epub 2014 Mar 21. J Bacteriol. 2014. PMID: 24659773 Free PMC article.
References
-
- Bourne, H.R., D.A. Sanders, and F. McCormick. 1991. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 349:117–127. - PubMed
-
- Connolly, T., and R. Gilmore. 1989. The signal recognition particle receptor mediates the GTP-dependent displacement of SRP from the signal sequence of the nascent polypeptide. Cell. 57:599–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

