An antigen-specific pathway for CD8 T cells across the blood-brain barrier
- PMID: 17682068
- PMCID: PMC2118703
- DOI: 10.1084/jem.20070064
An antigen-specific pathway for CD8 T cells across the blood-brain barrier
Abstract
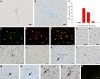
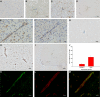
CD8 T cells are nature's foremost defense in encephalitis and brain tumors. Antigen-specific CD8 T cells need to enter the brain to exert their beneficial effects. On the other hand, traffic of CD8 T cells specific for neural antigen may trigger autoimmune diseases like multiple sclerosis. T cell traffic into the central nervous system is thought to occur when activated T cells cross the blood-brain barrier (BBB) regardless of their antigen specificity, but studies have focused on CD4 T cells. Here, we show that selective traffic of antigen-specific CD8 T cells into the brain occurs in vivo and is dependent on luminal expression of major histocompatibility complex (MHC) class I by cerebral endothelium. After intracerebral antigen injection, using a minimally invasive technique, transgenic CD8 T cells only infiltrated the brain when and where their cognate antigen was present. This was independent of antigen presentation by perivascular macrophages. Marked reduction of antigen-specific CD8 T cell infiltration was observed after intravenous injection of blocking anti-MHC class I antibody. These results expose a hitherto unappreciated route by which CD8 T cells home onto their cognate antigen behind the BBB: luminal MHC class I antigen presentation by cerebral endothelium to circulating CD8 T cells. This has implications for a variety of diseases in which antigen-specific CD8 T cell traffic into the brain is a beneficial or deleterious feature.
Figures



References
-
- Yeager, M.P., J.A. DeLeo, P.J. Hoopes, A. Hartov, L. Hildebrandt, and W.F. Hickey. 2000. Trauma and inflammation modulate lymphocyte localization in vivo: quantitation of tissue entry and retention using indium-111-labeled lymphocytes. Crit. Care Med. 28:1477–1482. - PubMed
-
- Carrithers, M.D., I. Visintin, S.J. Kang, and C.A. Janeway Jr. 2000. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 123:1092–1101. - PubMed
-
- Hickey, W.F., B.L. Hsu, and H. Kimura. 1991. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 28:254–260. - PubMed
-
- Irani, D.N., and D.E. Griffin. 1996. Regulation of lymphocyte homing into the brain during viral encephalitis at various stages of infection. J. Immunol. 156:3850–3857. - PubMed
-
- Krakowski, M.L., and T. Owens. 2000. Naive T lymphocytes traffic to inflamed central nervous system, but require antigen recognition for activation. Eur. J. Immunol. 30:1002–1009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

