Development of resistance in wild-type and hypermutable Pseudomonas aeruginosa strains exposed to clinical pharmacokinetic profiles of meropenem and ceftazidime simulated in vitro
- PMID: 17682103
- PMCID: PMC2043282
- DOI: 10.1128/AAC.00160-07
Development of resistance in wild-type and hypermutable Pseudomonas aeruginosa strains exposed to clinical pharmacokinetic profiles of meropenem and ceftazidime simulated in vitro
Abstract
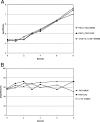
In this study we investigated the interplay of antibiotic pharmacokinetic profiles and the development of mutation-mediated resistance in wild-type and hypermutable Pseudomonas aeruginosa strains. We used in vitro models simulating profiles of the commonly used therapeutic drugs meropenem and ceftazidime, two agents with high levels of antipseudomonal activity said to have different potentials for stimulating resistance development. During ceftazidime treatment of the wild-type strain (PAO1), fully resistant mutants overproducing AmpC were selected rapidly and they completely replaced wild-type cells in the population. During treatment with meropenem, mutants of PAO1 were not selected as rapidly and showed only intermediate resistance due to the loss of OprD. These mutants also replaced the parent strain in the population. During the treatment of the mutator P. aeruginosa strain with meropenem, the slowly selected mutants did not accumulate several resistance mechanisms but only lost OprD and did not completely replace the parent strain in the population. Our results indicate that the commonly used dosing regimens for meropenem and ceftazidime cannot avoid the selection of mutants of wild-type and hypermutable P. aeruginosa strains. For the treatment outcome, including the prevention of resistance development, it would be beneficial for the antibiotic concentration to remain above the mutant prevention concentration for a longer period of time than it does in present regimens.
Figures


Similar articles
-
Potentiation of beta-lactams against Pseudomonas aeruginosa strains by Ro 48-1256, a bridged monobactam inhibitor of AmpC beta-lactamases.J Antimicrob Chemother. 1997 Sep;40(3):335-43. doi: 10.1093/jac/40.3.335. J Antimicrob Chemother. 1997. PMID: 9338484
-
Evolution of Pseudomonas aeruginosa Antimicrobial Resistance and Fitness under Low and High Mutation Rates.Antimicrob Agents Chemother. 2016 Jan 4;60(3):1767-78. doi: 10.1128/AAC.02676-15. Antimicrob Agents Chemother. 2016. PMID: 26729493 Free PMC article.
-
Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC.Antimicrob Agents Chemother. 2014 Jun;58(6):3091-9. doi: 10.1128/AAC.02462-13. Epub 2014 Mar 17. Antimicrob Agents Chemother. 2014. PMID: 24637685 Free PMC article.
-
Anti-biofilm and resistance suppression activities of CXA-101 against chronic respiratory infection phenotypes of Pseudomonas aeruginosa strain PAO1.J Antimicrob Chemother. 2010 Jul;65(7):1399-404. doi: 10.1093/jac/dkq143. Epub 2010 Apr 30. J Antimicrob Chemother. 2010. PMID: 20435779
-
Evolution and spread of antibiotic resistance.J Intern Med. 2002 Aug;252(2):91-106. doi: 10.1046/j.1365-2796.2002.01026.x. J Intern Med. 2002. PMID: 12190884 Review.
Cited by
-
Phages limit the evolution of bacterial antibiotic resistance in experimental microcosms.Evol Appl. 2012 Sep;5(6):575-82. doi: 10.1111/j.1752-4571.2011.00236.x. Epub 2012 Jan 13. Evol Appl. 2012. PMID: 23028398 Free PMC article.
-
Development and qualification of a pharmacodynamic model for the pronounced inoculum effect of ceftazidime against Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2009 Jan;53(1):46-56. doi: 10.1128/AAC.00489-08. Epub 2008 Oct 13. Antimicrob Agents Chemother. 2009. PMID: 18852268 Free PMC article.
-
The Saudi Thoracic Society guidelines for diagnosis and management of noncystic fibrosis bronchiectasis.Ann Thorac Med. 2017 Jul-Sep;12(3):135-161. doi: 10.4103/atm.ATM_171_17. Ann Thorac Med. 2017. PMID: 28808486 Free PMC article.
-
[Therapeutic drug monitoring and individual dosing of antibiotics during sepsis : Modern or just "trendy"?].Med Klin Intensivmed Notfmed. 2018 Mar;113(2):82-93. doi: 10.1007/s00063-016-0213-5. Epub 2016 Sep 13. Med Klin Intensivmed Notfmed. 2018. PMID: 27624768 Review. German.
-
Evaluation of Tobramycin and Ciprofloxacin as a Synergistic Combination Against Hypermutable Pseudomonas Aeruginosa Strains via Mechanism-Based Modelling.Pharmaceutics. 2019 Sep 12;11(9):470. doi: 10.3390/pharmaceutics11090470. Pharmaceutics. 2019. PMID: 31547301 Free PMC article.
References
-
- Alou, L., L. Aguilar, D. Sevillano, M. J. Gimenez, O. Echeverria, M. L. Gomez-Lus, and J. Prieto. 2005. Is there a pharmacodynamic need for the use of continuous versus intermittent infusion with ceftazidime against Pseudomonas aeruginosa? An in vitro pharmacodynamic model. J. Antimicrob. Chemother. 55:209-213. - PubMed
-
- Bowker, K. E., H. A. Holt, R. J. Lewis, D. S. Reeves, and A. P. MacGowan. 1998. Comparative pharmacodynamics of meropenem using an in-vitro model to simulate once, twice and three times daily dosing in humans. J. Antimicrob. Chemother. 42:461-467. - PubMed
-
- Bratu, S., D. Landman, J. Gupta, and J. Quale. 2007. Role of AmpD, OprF and penicillin-binding proteins in β-lactam resistance in clinical isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 56:809-814. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

