Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial
- PMID: 17682105
- PMCID: PMC2043273
- DOI: 10.1128/AAC.01086-06
Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial
Abstract
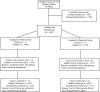
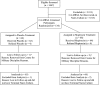
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) is an emerging pathogen that primarily manifests as uncomplicated skin and soft tissue infections. We conducted a cluster randomized, double-blind, placebo-controlled trial to determine whether targeted intranasal mupirocin therapy in CA-MRSA-colonized soldiers could prevent infection in the treated individual and prevent new colonization and infection within their study groups. We screened 3,447 soldiers comprising 14 training classes for CA-MRSA colonization from January to December 2005. Each training class was randomized to either the mupirocin or placebo study group, and the participants identified as CA-MRSA colonized were treated with either mupirocin or placebo. All participants underwent repeat screening after 8 to 10 weeks and were monitored for 16 weeks for development of infection. Of 3,447 participants screened, 134 (3.9%) were initially colonized with CA-MRSA. Five of 65 (7.7%; 95% confidence interval [95% CI], 4.0% to 11.4%) placebo-treated participants and 7 of 66 (10.6%; 95% CI, 7.9% to 13.3%) mupirocin-treated participants developed infections; the difference in the infection rate of the placebo- and mupirocin-treated groups was -2.9% (95% CI, -7.5% to 1.7%). Of those not initially colonized with CA-MRSA, 63 of 1,459 (4.3%; 95% CI, 2.7% to 5.9%) of the placebo group and 56 of 1,607 (3.5%; 95% CI, 2.6% to 5.2%) of the mupirocin group developed infections; the difference in the infection rate of the placebo and mupirocin groups was 0.8% (95% CI, -1.0% to 2.7%). Of 3,447 participants, 3,066 (89%) were available for the second sampling and completed follow-up. New CA-MRSA colonization occurred in 24 of 1,459 (1.6%; 95% CI, 0.05% to 2.8%) of the placebo group participants and 23 of 1,607 (1.4%; 95% CI, 0.05% to 2.3%) of the mupirocin group participants; the difference in the infection rate of the placebo and mupirocin groups was 0.2% (95% CI, -1.3% to 1.7%). Despite CA-MRSA eradication in colonized participants, this study showed no decrease in infections in either the mupirocin-treated individuals or within their study group. Furthermore, CA-MRSA eradication did not prevent new colonization within the study group.
Figures
Similar articles
-
Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers.Clin Infect Dis. 2004 Oct 1;39(7):971-9. doi: 10.1086/423965. Epub 2004 Sep 2. Clin Infect Dis. 2004. PMID: 15472848
-
Use of intranasal mupirocin to prevent methicillin-resistant Staphylococcus aureus infection in intensive care units.Crit Care. 2005 Jun;9(3):R246-50. doi: 10.1186/cc3512. Epub 2005 Mar 31. Crit Care. 2005. PMID: 15987397 Free PMC article.
-
Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients.Infect Control Hosp Epidemiol. 2007 Oct;28(10):1155-61. doi: 10.1086/520102. Epub 2007 Aug 1. Infect Control Hosp Epidemiol. 2007. PMID: 17828692 Clinical Trial.
-
Agents for the decolonization of methicillin-resistant Staphylococcus aureus.Pharmacotherapy. 2009 Mar;29(3):263-80. doi: 10.1592/phco.29.3.263. Pharmacotherapy. 2009. PMID: 19249946 Review.
-
Intranasal mupirocin prophylaxis in elective surgery. A review of published studies.Chemotherapy. 2008;54(1):9-16. doi: 10.1159/000112312. Epub 2007 Dec 7. Chemotherapy. 2008. PMID: 18063862 Review.
Cited by
-
Profiling of serum factors associated with Staphylococcus aureus skin and soft tissue infections as a foundation for biomarker identification.Front Immunol. 2023 Nov 20;14:1286618. doi: 10.3389/fimmu.2023.1286618. eCollection 2023. Front Immunol. 2023. PMID: 38054000 Free PMC article.
-
Prevention of Recurrent Staphylococcal Skin Infections.Infect Dis Clin North Am. 2015 Sep;29(3):429-64. doi: 10.1016/j.idc.2015.05.007. Infect Dis Clin North Am. 2015. PMID: 26311356 Free PMC article. Review.
-
Staphylococcus aureus colonization of healthy military service members in the United States and Afghanistan.BMC Infect Dis. 2013 Jul 16;13:325. doi: 10.1186/1471-2334-13-325. BMC Infect Dis. 2013. PMID: 24060181 Free PMC article.
-
Impact of MRSA on the Military Medical Service and Diagnostic Point-of-Care Options for the Field Setting.Eur J Microbiol Immunol (Bp). 2018 Jun 20;8(2):31-33. doi: 10.1556/1886.2018.00012. eCollection 2018 Jun 25. Eur J Microbiol Immunol (Bp). 2018. PMID: 29997908 Free PMC article. Review.
-
Opportunities and Obstacles in the Prevention of Skin and Soft-Tissue Infections Among Military Personnel.Mil Med. 2019 Nov 1;184(Suppl 2):35-43. doi: 10.1093/milmed/usz105. Mil Med. 2019. PMID: 31778193 Free PMC article.
References
-
- Baggett, H. C., T. W. Hennessy, K. Rudolph, D. Bruden, A. Reasonover, A. Parkinson, R. Sparks, R. M. Donlan, P. Martinez, K. Mongkolrattanothai, and J. C. Butler. 2004. Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska, 1996-1998. J. Infect. Dis. 189:1565-1573. - PubMed
-
- Buckingham, S. C., L. K. McDougal, L. D. Cathey, K. Comeaux, A. S. Craig, S. K. Fridkin, and F. C. Tenover. 2004. Emergence of community-associated methicillin-resistant Staphylococcus aureus at a Memphis, Tennessee children's hospital. Pediatr. Infect. Dis. J. 23:619-624. - PubMed
-
- Centers for Disease Control and Prevention. 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus-Minnesota and North Dakota, 1997-1999. Morb. Mortal. Wkly. Rep. 48:707-710. - PubMed
-
- Centers for Disease Control and Prevention. 2003. Methicillin-resistant Staphylococcus aureus infections among competitive sports participants- Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000-2003. Morb. Mortal. Wkly. Rep. 52:793-795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

